Bronchiectasis can result from many diseases, which makes the aetiological investigation a complex process demanding special resources and experience. The aetiological diagnosis has been proven to be useful for the therapeutic approach.
ObjectiveEvaluate how accurately and extensive the clinical and aetiological research was for adult bronchiectasis patients in pulmonology outpatient service which were not following a pre-existing protocol.
MethodsWe retrospectively reviewed the records of 202 adult patients with bronchiectasis, including the examinations performed to explain the aetiology.
ResultsThe mean age of the patients was 54±15 years, there was a predominance of female (63.9%) and non-smoker (70%) patients. Functional evaluation showed a mild airway obstruction.
The sputum microbiological examination was available for 168 patients (43.1% had 3 or more sputum examinations during one year). Immunoglobulins and α1-antitrypsin were measured in around 50% of the patients. The sweat test and the CF genotyping test were performed in 18% and 17% of the patients, respectively.
The most commonly identified cause was post-infectious (30.3%), mostly tuberculosis (27.2%). No definitive aetiological diagnosis was established in 57.4% of the patients. We achieved a lower aetiological diagnosis if we compare our series with studies in which a diagnostic algorithm was applied prospectively.
ConclusionsThe general characteristics of our patients were similar with other series. Detailed investigation of bronchiectasis is not a standard practice in our outpatient service. These results suggest that the use of a predefined protocol, based on current guidelines, could improve the assessment of these patients and facilitate the achievement of a definitive aetiology.
Bronchiectasis (BE) results from a large number of diseases and is associated with high morbidity and significant costs for health-care systems.1,2
For many years respiratory infections were the main identifiable cause. However, post-infectious BE has been decreasing mainly in developed countries due to vaccination programmes, antibiotic therapy and better social-hygienic conditions while other congenital or acquired causes have been described now that we have more accurate diagnosis methods.3
In recent years, a number of studies have shown that an aetiological diagnosis can change the therapeutic approach in a relevant percentage of patients.4–6
Research carried out in units with great experience in BE and with specialized resources may not be typical of the assessment which is carried out in the majority of general respiratory services. There is some evidence that the study and follow-up of patients in specialized centres probably contribute to a larger number of aetiological diagnoses and more appropriate treatment.5,6
The local characteristics of BE patients were unknown, namely the level of research, BE aetiologies, functional severity and microbiological characteristics.
The aim of this study was to evaluate how accurate and extensive the clinical and aetiological research was for BE patients followed in the pulmonology outpatient service of a central hospital which did not routinely use a thorough, pre-existing protocol for it.
Materials and methodsA retrospective analysis of the clinical records of adult patients with BE diagnosis who were seen and managed in the pulmonology outpatient service, from January 2008 to January 2009, was carried out. The patients concerned had to be followed-up for at least 1 year.
The diagnosis of BE was established by the characteristic features of the high-resolution computed tomography (HRCT) of the thorax (bronchoarterial ratio ≥1, dilated bronchi visible ≤1cm from parietal pleura, cystic changes and a lack of normal bronchial tapering) or the CT, if unequivocal evidence of BE existed.
Patients with CF and interstitial pathology were excluded.
Each pulmonologist involved was totally responsible for the clinical records, the follow-up and aetiological research, without having to comply with any predefined protocol. We arrived at an aetiological diagnosis based on the clinical records and the results of earlier diagnostic tests done by the patient's clinician. There was no subsequent research done to complement our study.
The demographical, clinical, functional, radiological and microbiological data were reviewed. The exams that had been performed to explain the BE aetiology, namely, the sweat test, cystic fibrosis (CF) genotyping, measurement of serum immunoglobulins (Igs), α1-antitrypsin (AAT) and the semen analysis were recorded.
The sweat test was performed by conductivity method and the CF genotyping test screened the 33 most frequent CFTR mutations in Portuguese population. In some cases a complete analysis of the CFTR gene was performed.
The respiratory function tests were done according to standard international recommendations.7
The isolation of the same pathogen in at least 3 sputum samples, for at least one year and with a minimal interval of one month, was considered by the authors as the definition of chronic airway colonization.
Statistical analysis was done with SPSS 19, 2010 software program (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA). Descriptive statistics (mean, standard deviation) were used to summarize the data. The Kolmogorov–Sminorv test was used to analyze the distribution of variables. Variables with a normal distribution were analyzed using the student t-test, while in the abnormal ones the Mann–Whitney U test was employed. The significance level was set at p<0.05.
The project was approved by the Ethics Committee for Local Health of the CHSJ.
ResultsThe clinical records of 202 patients with BE diagnosis were reviewed.
The diagnosis was established by HRCT in 94% of the patients and by CT scan in the remaining patients.
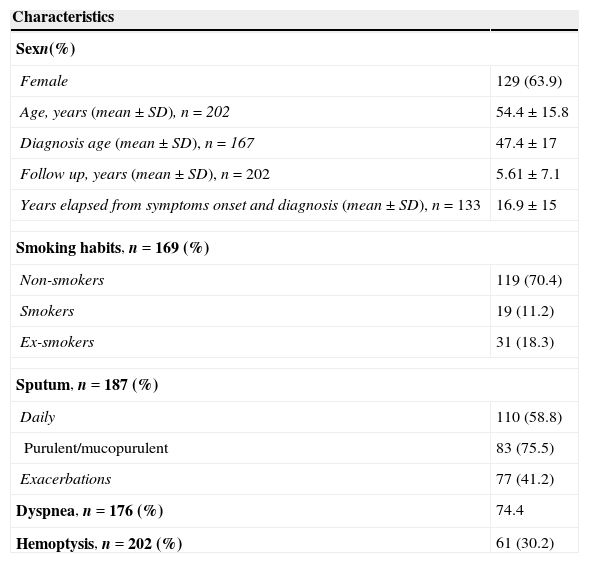
The patients’ general characteristics are shown in Table 1.
General characteristics.
| Characteristics | |
|---|---|
| Sexn(%) | |
| Female | 129 (63.9) |
| Age, years (mean±SD), n=202 | 54.4±15.8 |
| Diagnosis age (mean±SD), n=167 | 47.4±17 |
| Follow up, years (mean±SD), n=202 | 5.61±7.1 |
| Years elapsed from symptoms onset and diagnosis (mean±SD), n=133 | 16.9±15 |
| Smoking habits, n=169 (%) | |
| Non-smokers | 119 (70.4) |
| Smokers | 19 (11.2) |
| Ex-smokers | 31 (18.3) |
| Sputum, n=187 (%) | |
| Daily | 110 (58.8) |
| Purulent/mucopurulent | 83 (75.5) |
| Exacerbations | 77 (41.2) |
| Dyspnea, n=176 (%) | 74.4 |
| Hemoptysis, n=202 (%) | 61 (30.2) |
Fertility information was missing for 40 (54.7%) of the male patients. The upper airway symptoms were also rarely mentioned, as well as, occupational exposure to toxic chemicals, family history of BE, non respiratory symptoms and consanguinity data.
RadiologyBE had a bilateral distribution in 89% of the patients. In 6% of the cases only 1 lobe was affected and in 80.4% 4 or more lobes were affected or there were cystic changes in 2 or more lobes. The predominant morphology was cylindrical (47%), followed by cylindrical associated with cystic (34%). The lower lobes (LL) were the most frequently affected (right LL in 88% and left LL in 86%).
MicrobiologyThirty-four patients (16.8%) did not have a record of a sputum microbiological examination.
Out of 168 patients with at least one sputum microbiological examination, the most frequently isolated bacteria once or intermittently was Haemophilus influenzae (26.8%), followed by Streptococcus pneumoniae (16.7%) and Pseudomonas aeruginosa (14.9%).
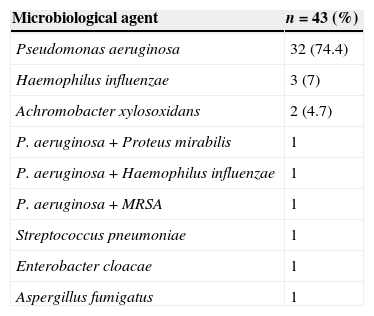
Eighty-seven patients (43.1%) had done 3 or more sputum examinations during the period of one year, of which 43 (49.4%) met the chronic colonization criteria. The most commonly isolated bacteria in this set of patients was P. aeruginosa (81.4%), associated in 3 cases with other bacteria, followed by H. influenzae (9.1%) and Achromobacter xylosoxidans (4.7%) (Table 2).
Chronic airway colonization.
| Microbiological agent | n=43 (%) |
|---|---|
| Pseudomonas aeruginosa | 32 (74.4) |
| Haemophilus influenzae | 3 (7) |
| Achromobacter xylosoxidans | 2 (4.7) |
| P. aeruginosa+Proteus mirabilis | 1 |
| P. aeruginosa+Haemophilus influenzae | 1 |
| P. aeruginosa+MRSA | 1 |
| Streptococcus pneumoniae | 1 |
| Enterobacter cloacae | 1 |
| Aspergillus fumigatus | 1 |
MRSA, methicilin-resistant Staphylococcus aureus.
Atypical mycobacteria were identified in 20 patients, corresponding to 23 different isolations, as 2 different species were isolated in 3 patients on different occasions. The species belonging to Mycobacterium avium-complex (MAC) were the most common (65.2%), followed by M. kansasii (17.4%), M. gordonae (8.7%), M. fortuitum (4.3%) and M. peregrinum (4.3%). In most cases (60.9%) only one positive culture was identified. Ten patients underwent treatment, 9 for MAC and 1 for M. Kansasii infection, of which 2 did not meet the nontuberculosis mycobacterial infection published criteria.8
After the BE diagnosis, 5 patients had pulmonary tuberculosis (PT).
Respiratory functionThe results of the functional assessments, based on the most recent records, are presented in Table 2. Overall, the FEV1 was 65% of predicted, of which 32% had FEV1 <50%, 31% had between 50 and 70% and 37% reached >70%.
Thirteen percent of the patients had type II and 5% type I respiratory failure.
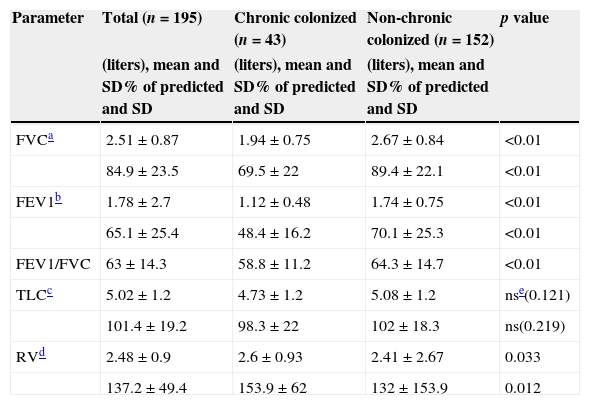
In a comparative analysis of pulmonary function of chronic and non-chronic colonized patients the figures (Table 3) showed statistically significant lower values of FVC, FEV1, FEV1/FVC ratio and RV in colonized patients. The differences between the TLC did not reach statistical significance.
Pulmonary function.
| Parameter | Total (n=195) | Chronic colonized (n=43) | Non-chronic colonized (n=152) | p value |
|---|---|---|---|---|
| (liters), mean and SD% of predicted and SD | (liters), mean and SD% of predicted and SD | (liters), mean and SD% of predicted and SD | ||
| FVCa | 2.51±0.87 | 1.94±0.75 | 2.67±0.84 | <0.01 |
| 84.9±23.5 | 69.5±22 | 89.4±22.1 | <0.01 | |
| FEV1b | 1.78±2.7 | 1.12±0.48 | 1.74±0.75 | <0.01 |
| 65.1±25.4 | 48.4±16.2 | 70.1±25.3 | <0.01 | |
| FEV1/FVC | 63±14.3 | 58.8±11.2 | 64.3±14.7 | <0.01 |
| TLCc | 5.02±1.2 | 4.73±1.2 | 5.08±1.2 | nse(0.121) |
| 101.4±19.2 | 98.3±22 | 102±18.3 | ns(0.219) | |
| RVd | 2.48±0.9 | 2.6±0.93 | 2.41±2.67 | 0.033 |
| 137.2±49.4 | 153.9±62 | 132±153.9 | 0.012 |
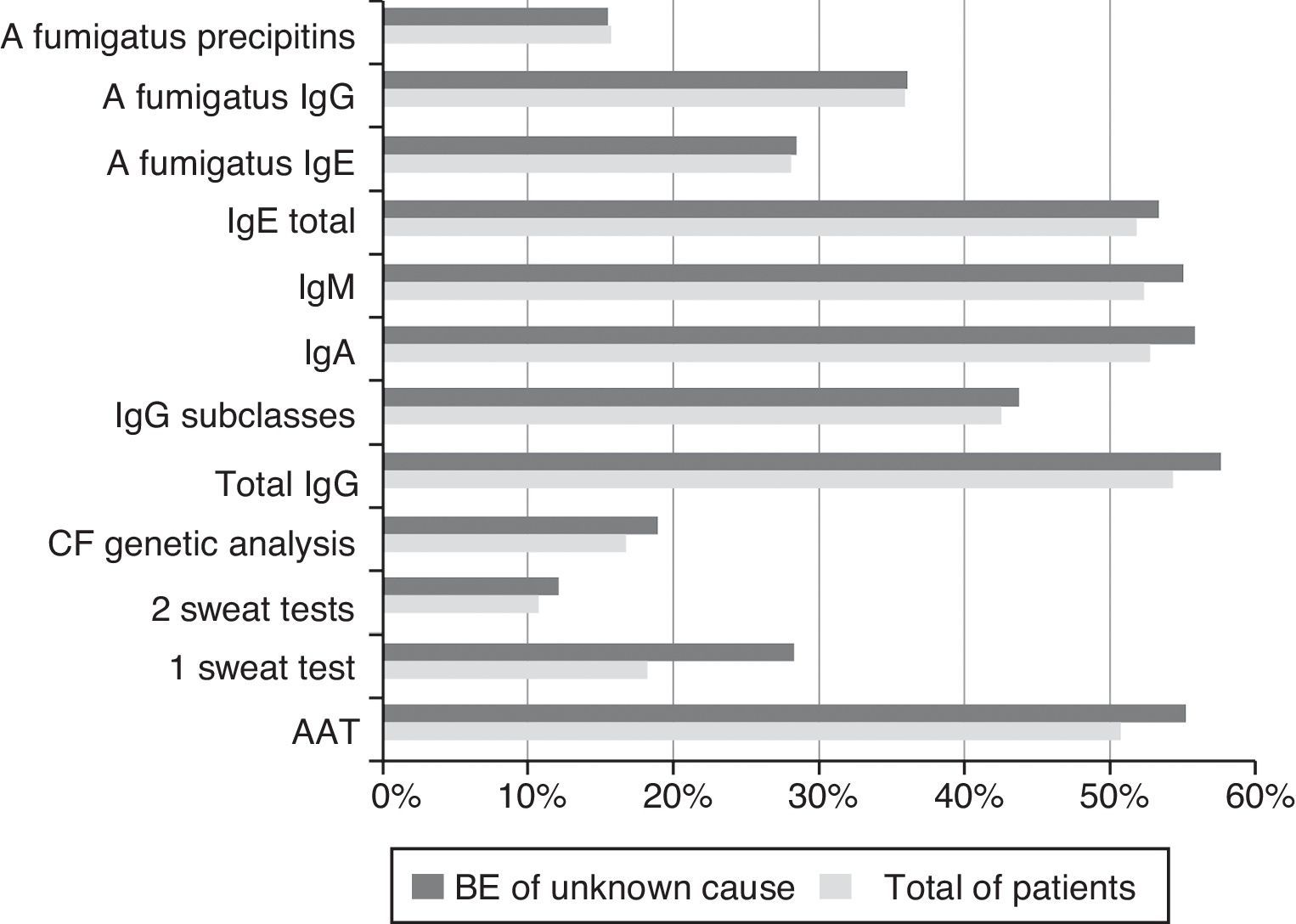
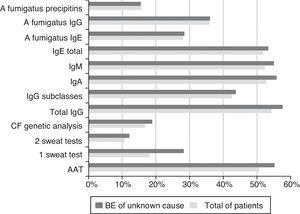
The exams carried out to investigate the BE aetiology are shown in Fig. 1.
The CF genetic analysis was performed in 34 (16.8%) of the cases, and 1 patient was found to have 1 mutation (R334W). The extensive genetic test was performed in 4.9% of the patients. One mutation (V754M) was detected in 1 of the patients and 2 mutations [G576A; R668C] were detected in another patient which after family screening was confirmed to be in cis. The sweat test in these patients was negative.
Five patients did a semen analysis, and infertility was confirmed in 3 cases (one was diagnosed as PCD and the other two were considered as of unknown cause).
In relation to the upper airways, 45 patients (22.3%) did nasal CT and findings were suggestive of chronic sinusitis in 24 (11.9%).
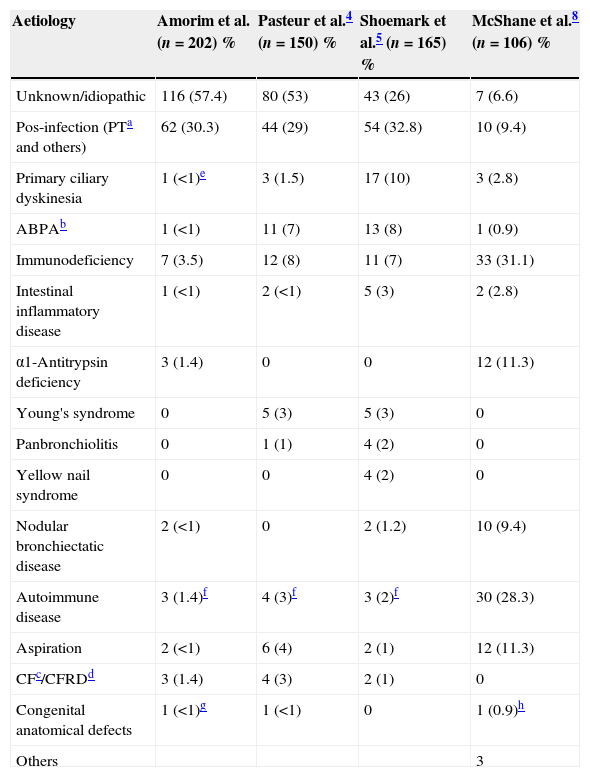
The aetiologies shown in Table 4 were based on the clinical records and the results of the exams carried out. A definitive aetiological diagnosis was not established in 57.4% of the patients. In 30.3% BE was post-infectious, mostly secondary to tuberculosis (27.2%).
Aetiological diagnosis and comparison with prospective studies.
| Aetiology | Amorim et al. (n=202) % | Pasteur et al.4 (n=150) % | Shoemark et al.5 (n=165) % | McShane et al.8 (n=106) % |
|---|---|---|---|---|
| Unknown/idiopathic | 116 (57.4) | 80 (53) | 43 (26) | 7 (6.6) |
| Pos-infection (PTa and others) | 62 (30.3) | 44 (29) | 54 (32.8) | 10 (9.4) |
| Primary ciliary dyskinesia | 1 (<1)e | 3 (1.5) | 17 (10) | 3 (2.8) |
| ABPAb | 1 (<1) | 11 (7) | 13 (8) | 1 (0.9) |
| Immunodeficiency | 7 (3.5) | 12 (8) | 11 (7) | 33 (31.1) |
| Intestinal inflammatory disease | 1 (<1) | 2 (<1) | 5 (3) | 2 (2.8) |
| α1-Antitrypsin deficiency | 3 (1.4) | 0 | 0 | 12 (11.3) |
| Young's syndrome | 0 | 5 (3) | 5 (3) | 0 |
| Panbronchiolitis | 0 | 1 (1) | 4 (2) | 0 |
| Yellow nail syndrome | 0 | 0 | 4 (2) | 0 |
| Nodular bronchiectatic disease | 2 (<1) | 0 | 2 (1.2) | 10 (9.4) |
| Autoimmune disease | 3 (1.4)f | 4 (3)f | 3 (2)f | 30 (28.3) |
| Aspiration | 2 (<1) | 6 (4) | 2 (1) | 12 (11.3) |
| CFc/CFRDd | 3 (1.4) | 4 (3) | 2 (1) | 0 |
| Congenital anatomical defects | 1 (<1)g | 1 (<1) | 0 | 1 (0.9)h |
| Others | 3 |
The 7 cases of immunodeficiency include 2 IgA deficiencies (with IgG4 deficiency), 3 common variable immunodeficiencies and 2 hematologic malignancies.
DiscussionThe 202 patients in the study were followed in the pulmonology outpatient service. There was a predominance of female and non-smoker patients in the evaluated population, which is consistent with most published series.9,10
There was a long delay in diagnosis. This could be due to the lack of specificity of BE symptoms which, on many occasions, can lead to a misdiagnosis of COPD or asthma11,12 with prognostic implications.4,5,13
The patient information was recorded and follow-ups carried out without a predefined protocol, based on existing guidelines, which certainly contributed to the absence of important clinical historic data, subjective descriptions of some symptoms and the lack of appropriate investigation tests.
The result of the functional assessment was compatible with an obstructive pattern, as described in the literature.14,15
The HRCT is considered the gold standard diagnostic test for BE and in this review it was chosen as the confirmatory diagnostic method. In most cases BE was bilateral and the predominant morphology was cylindrical, followed by cylindrical in association with cystic. This result is partly a consequence of a more generalized use of HRCT and its high sensitivity.16,17
According to current recommendations, sputum culture should be carried out regularly.10,18 It was not quite clear why 115 (56.9%) patients had not had one or had fewer than 3 samples per year. This may have led to an underestimation of the real number of chronic colonizations. The bacteria most commonly isolated, intermittently or only once, was H. influenzae and the bacteria most frequently associated with chronic colonization was P. aeruginosa. The presence of the latter has been linked to greater clinical and functional severity, greater numbers of exacerbations and worse quality of life.2,19 This fact could explain why further bacteriological sputum tests had been requested for this subset of patients, which means that P. aeruginosa may not be the bacteria most frequently associated with chronic colonization.
The pulmonary function was significantly worse in the chronic colonized patients, which reflects the severity usually associated with the chronic infection.
The prevalence of mycobacteria in BE patients is relatively low, ranging between 2% and 10%.20,21 In this series the prevalence was in this range, MAC being the most common.22
The assessment of BE patients must always include the investigation of the primary cause.10,18,23
Fig. 1 shows the diagnostic tests carried out in total patients and in the cases of BE of unknown cause. The percentage of tests done is similar between the two groups, which suggests that further research could be done, using our local resources.
According to current recommendations, a conductivity sweat test value >60mmol/l should be confirmed by chloride measurement24; this is not available in our hospital.
The CF genetic test allowed the detection of mutations in 3 patients who were considered to have a CFTR-related disorder because the sweat test was negative and only one mutation or two mutations in cis were detected.25,26 However, to exclude CF with accuracy the quantitative sweat test should have been carried out on these patients and, if necessary, the nasal potential difference, which is not done in our country.
In this review, an aetiological diagnosis was obtained in 42.1% (Table 4) and the post-infectious cause was the most common, like other published series.2,14,27 Compared with studies in which a diagnostic algorithm was applied prospectively (Table 4) our result is lower.4,5,28
COPD was not considered as a cause of BE, despite the fact that some studies have shown an unexpectedly high incidence of BE in this disease.28–31 In light of current knowledge,10 BE associated with COPD should only be established after a careful aetiological work-up to exclude other associated diseases.
The reduced percentage or even absence of some pathologies in our study, namely ABPA, immunodeficiency and PCD4–6 suggest that the implementation of an algorithmic investigation and the availability of specific tests could facilitate the achievement of rare and/or complex diagnosis.
ConclusionIn the recent years there has been a resurgence of interest in BE and it is urgent to set out our practice in the latest existing guidelines and to review our standards of care.
In our country there are no specialized centres for BE; not even in central hospitals.
As BE can result from several diseases, a detailed clinical history and physical examination is fundamental in order to establish the diagnostic hypothesis and to guide the research in the most appropriate way.
Our results show how important, useful clinical and aetiological findings could be missed when a pre-existing protocol is not being used. If the investigation and medical follow-ups were done with a pre-defined protocol based on current published guidelines,10,18 we would probably have a higher percentage of accurate diagnoses and a better clinical characterization of the patients.
The establishment of dedicated outpatient clinics may help to improve the global management of these patients, from diagnosis to therapeutic interventions, and consequently improve prognosis and understanding of the natural course of the disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document
Conflicts of interestDr. Javier de Gracia declare the following conflicts of interest: received industry-sponsored grand funding from Grifolds, Gilead, Novartis for participating in clinical trials and received fees for lectures and other educational activities from Praxis.
I would like to thank the collaboration of all colleagues from the Pneumology Service who referred patients, accompanied in outpatient care, to be included in the investigation.