Catathrenia is a rare sleep disorder first described by Roeck et al.1 Originally classified as a parasomnia, it is currently included within the group of respiratory sleep disorders. Its incidence, prevalence, and physiopathology are unknown, and its onset is more frequent in adolescents and young adults of average weight. Bed partners are the ones who commonly report strange sounds while breathing during sleep, as affected individuals are unaware of their problem.
Catathrenia usually occurs during REM sleep, though it may be also present in other stages. A catathenia event is characterized by a repeated groaning or moaning sound during prolonged expiration preceded by a deep inhalation and accompanied by a breathing pattern marked by bradypnea of variable duration. Its clinical relevance remains uncertain; catathrenia is considered a self-limited benign condition causing no negative effects beyond significant social nuisance. Although still debated, a significant proportion of patients with catathrenia do report disrupted sleep and some sleepiness or tiredness as in our current case.2 There is little documented experience in treating the condition, but some studies have shown resolution of events with continuous positive airway pressure (CPAP). Here we describe a patient with substantial clinical repercussions due to sleep fragmentation, responding favorably to CPAP without a need for treatment at high-pressure levels.
Case reportA 22-year-old average-weight (BMI 24) woman with no relevant medical history who did not smoke or drink complained of nocturnal groaning and arousals due to the noise which caused sleep fragmentation, low quality of sleep, daytime tiredness, and headache predominantly in the morning hours. An examination was performed on the patient, including neurologic and otolaryngology assessment and pulmonary function examination, revealing no pathological findings that could explain the clinical symptoms.
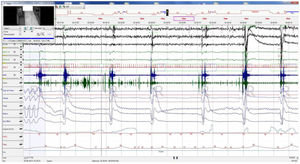
A polysomnography (PSG) was carried out showing apnea/hypopnea index (AHI) of 4.8e/h, desaturation index of 0e/h, and respiratory disturbance index (RDI) of 14e/h. The PSG revealed 18 cathathrenia events, most of which occurred from REM sleep. These events were characterized by prolonged expiration with acute sound during bradypnea without oxygen desaturation, lack of effort in the chest and abdomen, and sleep fragmentation related to the electroencephalographic arousals secondary to these events (Fig. 1). The results of the study confirmed the diagnosis of catathrenia. Based on the clinical impact, CPAP treatment was administered at a pressure of 4 cmH2O.
Baseline polysomnography: This figure evidences nocturnal characteristic bradypnea without effort in the chest/abdomen or oxygen desaturation. Associated electroencephalographic (EEG) arousals during stage REM. EEG channels (from top to bottom): C4/A1, C3/A2, O2/A1, EOG1/A1, EOG2/A1, ECG, EMG, snore, flow, thermistor, chest band, abdominal band, sum of band readings, phase angle, oxygen saturation, heart rate and position.
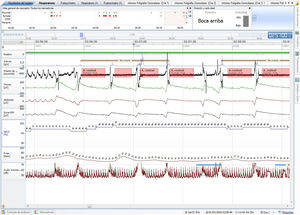
To confirm treatment results, a respiratory polygraphy test with a microphone was performed on 2 consecutive nights. We conducted a baseline polygraphy test (Fig. 2) which detected catathrenia noise with the typical signs in flow/effort bands without desaturation. In the following polygraphy with a low CPAP pressure setting (4 cmH2O) minimal residual events were observed.
Baseline polygraphy: EEG channels (top to bottom): position, activity, flow, chest band, abdominal band, sum of band readings, oxygen saturation, heart rate, and microphone. There is noise all through the events (microphone channel). Following each event there is an increase in activity signal.
Clinical response to CPAP was excellent; she has improved her daytime sleepiness and remains free of symptoms showing a good compliance after 24 months.
DiscussionWe present the case of a patient with substantial clinical repercussions stemming from catathrenia. A sleep study confirmed sleep fragmentation secondary to catathrenia events (deep inspiration followed by a prolonged expiration associated with a groaning, followed by a brief expiration and deep inspiration) as evidenced by the fact that each event was associated with an electroencephalographic (EEG) arousal. For the sake of description, we used the methods appearing in the case series of Guilleminault et al. in which only those sleep epochs with 2 or more events were included in the analysis.3
Our patient had 18 epochs with at least 2 breaths marked by catathrenia, and another 7 breaths did not meet the aforementioned criteria. Although the total duration was short (9 min for the entire reading), these events involved marked sleep fragmentation and poor sleep quality. The respiratory events do not sufficiently explain the symptoms in this patient, as AHI and desaturation index were normal, and an RDI of 14 was only reached following flow limitation. Additional tests were carried out, including pulmonary function test and neurological and otolaryngology assessment; these did not evidence any type of anatomical or other abnormality such as those described by Zeliang et al.4
Given the clinical repercussions caused by the condition, a therapeutic test consisting of CPAP at a pressure of 4 cm H2O was performed. Although substantially higher-pressures are recommended in the literature,3,6 a substantial improvement was achieved in our patient. Guilleminault et al.3 showed that their patient’s groaning responded completely using CPAP (higher-pressure settings: 7–10 cmH20). These pressures were pursued to eliminate flow limitation (abnormal RDI was present in these series of cases) but pleased to find the resolution of catathrenia events. In our case, a second sleep study was carried out to confirm response; in this case polygraphy was used, as in the report of Romigi et al.,5 this procedure may be useful for screening and diagnosis of this pathology when there is high suspicion of catathrenia. The NOX-T3 sleep monitor, which is equipped with a directional microphone, was used for polygraphy studies. The test was performed on 2 consecutive nights in order to compare the first night (baseline) to the second, during which a low CPAP pressure setting was administered. The length of both studies was comparable.
A significant number of events secondary to catathrenia were observed on the first night, with each of these 38 events leading to body movements that can suggest arousals (no confirmation is available since we lack EEG in this test). AHI and RDI were slightly lower than the observed in the PSG (AHI 3e/h, RDI 12e/h). The following night with the lowest-pressure settings of CPAP we observed that almost all catathenia events disappeared; there was a substantial reduction in the number of events from 38 to 8 events with improvement in clinical presentation despite persistence of AHI of 2e/h and RDI of 9e/h. Treating catathrenia events associated with abnormal RDI usually leads to the increased pressures described by Guilleminault et al.3 It remains controversial if titrating CPAP to eliminate flow limitation may be associated with improved clinical outcomes compared to treating apneas or hypopneas.7 We believe our patient, despite her young age, has remained compliant with CPAP for over two years due to the clinical benefit produced at the lowest pressure settings of CPAP. There are other studies were catathrenia was treated with selected soft tissue surgeries of the upper airway or with mandibular advancement device.8
In summary, catathrenia is an uncommon disorder characterized by a distinct breathing pattern in which CPAP treatment seems to be effective but its utility is limited by poor patients acceptability. Setting CPAP pressures to control cathatrenia events (leaving aside flow limitation) could be related to better CPAP compliance.
Author’s contributionsTG and AC were responsible for the conception and design of the study, and wrote and edited the manuscript. MP-C, PR, MF-T and FE contributed to the drafting and revision of the manuscript. All authors read and approved the final manuscript.
FundingThis study was undertaken without funding.
Conflicts of interestWe wish to confirm that are no known conflicts of interest associated with this publication and there has been no financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial manager and direct communications with the office). She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept e-mail from ana.casal.mourino@sergas.es
We confirm that the manuscript is not published elsewhere, in any language, and is not under simultaneous consideration by any other journal. Signed by all authors as follows:
Teresa Gómez
Ana Casal
María del Pilar Carballosa De Miguel
Paula Rodríguez Rodríguez
María Fernanda Troncoso Acevedo
Farah Ezzine De Blas