To characterise the morbidity of COPD out-patients based on symptoms, acute exacerbations, FEV1 and comorbidities, and to explore the association between different patients’ characteristics such as social, demographic, clinical history or exposure.
MethodsStable COPD outpatients over 40 years old diagnosed according to GOLD criteria were included consecutively; the exclusion criteria were only refusal to participate and inability to understand clinical questionnaires. A survey of demographic and clinical data was conducted. Symptoms were evaluated using the CAT and mMRC questionnaires. The number of COPD acute exacerbations reported in the previous year was assessed, and spirometry performed on all participants according to ATS/ERS recommendations. Different variables were collected and then related to each other.
ResultsWe studied 303 COPD outpatients, all Caucasians, 79.5% males and mostly elderly. 65.7% of participants reported having low monthly income and 87.8% a low education level. Tobacco smoking was the most common exposure identified but a substantial proportion of COPD patients were non-smokers (26%). Frequent acute exacerbations were reported by 38.0% of patients. The mean post-bronchodilator FEV1 was 53.2%. The distribution of patients according to GOLD 2017 stage and classification was respectively 9.9%, 41.9%, 35.0% and 13.2% from 1 to 4 and 23.1%, 39.6%, 2.3% and 35.0% from GOLD A to D. Only 29 patients (9.5%) presented no comorbid conditions, and the most common were hypertension, heart diseases and dyslipidaemia.
ConclusionsOur data confirms COPD as a complex and heterogeneous disorder, with a significant morbidity due to the nature of symptoms, frequent comorbidities and exacerbations. A substantial proportion of COPD patients were never-smokers, mainly women, calling attention to the need for COPD recognition in these cases. COPD in women, in never-smokers and in patients with a previous diagnosis of asthma presented some specific characteristics. Some patient characteristics are associated with frequent acute exacerbations. FEV1 was strongly related both to symptoms and exacerbations.
Chronic Obstructive Pulmonary Disease is the 4th leading cause of death and the most common chronic respiratory disease worldwide. In developed countries there is a growing recognition of COPD as a cause of mortality and disability, despite significant improvements in life expectancy and lower death rates in many diseases. COPD currently represents one of the most significant health problems globally,1 and its economic and social impact is constantly increasing.2 Despite the significant burden of COPD, there is little published literature concerning the characterisation of the disease in Portuguese patients.3 However, population-based studies are the mainstay for health planning and economic investment for any chronic respiratory diseases.4 The aim of this study was (1) to characterise the morbidity of COPD outpatients based on symptoms, acute exacerbations, FEV1 and comorbidities, and (2), and to explore the association between different patient characteristics such as social, demographic, clinical history or exposure.
Materials and methodsA cross-sectional study on COPD was conducted in the ambulatory pulmonary clinic of Hospital de Guimarães, Portugal, between March 2016 and May 2017. Stable COPD patients over 40 years of age, diagnosed according to GOLD criteria, were included consecutively after giving their written informed consent.5 Exclusion criteria were the refusal to participate or inability to understand simple questionnaires. The study was approved by the Hospital de Guimarães Ethics Committee, the Research Ethics Committee of Minho University and the Portuguese Data Protection Agency. We followed the STROBE guidelines for reporting observational studies.6
A questionnaire of demographic and clinical data and the Graffar Social Classification,7 validated for use in Portuguese population, was used. Symptoms were evaluated using the Portuguese versions of the COPD Assessment Test (CAT) and the Medical Research Council Dyspnoea Questionnaire (mMRC). Occupational exposure to dust, gas or fumes relevant to COPD were self-reported, and dichotomised as regularly exposed or not-regularly exposed. Indoor exposure to household air pollution from coal and biomass fuel combustion was considered only for regular exposure to cooking, and also dichotomised as regularly exposed and not-regularly exposed. The number of COPD acute exacerbations (ECOPD) reported in the previous year and the patients’ comorbidities were evaluated by using the hospital data base, the health data platform, or patient self-report. We defined ECOPD, according to GOLD, as an acute worsening of respiratory symptoms that result in additional therapy, but also require an unplanned medical visit. All participants performed spirometries according to the American Thoracic Society and the European Respiratory Society recommendations for standardised lung function testing,8,9 and referenced according to Global Lung Function Initiative predict equations (GLI 2012).10 It should be noted that, if we had used a post-bronchodilator FEV1/FVC >lower limit of normal (LLN) instead of the fixed value suggested by the GOLD to define airflow limitation, COPD would have been ruled out in 46 patients (15.2%)
Statistical analysisStatistical analysis was performed with IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY: IBM Corp. Independence for categorical variables was analysed with Chi-square Test and a statistical significance was considered when p<0.05. Binary logistic modelling was performed to analyse predictors’ significance and effect size in dichotomous data.
ResultsPatients’ characteristicsA total of 344 participants were recruited. Five refused to participate, eight were unable to understand clinical questionnaires and COPD was later ruled-out for 28 subjects. We studied 303 COPD outpatients, all Caucasians, predominantly married (77.6%), retired (84.1%) and living with their families (89.8%). Many different exposures relevant for COPD overlapped in the same patients: 121 (39.9%) smoker or ex-smoker patients also reported occupational exposure. Tobacco smoking was the most common exposure identified in 74.0% of patients (86.7% of men and 24.2% of women), 18.2% being current smokers and 55.8% ex-smokers. Self-reported occupational exposure to gas, fumes and dust, either mineral or biological, relevant to COPD, were reported by 55.5% of patients, and sustained indoor-exposure to household air pollution from coal and biomass fuel combustion was reported by 63.8% of women. The most important demographic, clinical and functional characteristics are presented in Table 1.
Demographic, clinical and functional characteristics of COPD patients.
| Characteristics | n=303 |
|---|---|
| Male gender | 241 (79.5) |
| Mean age (years) | 67.5±10.2 |
| Age ≥65 years | 186 (61.4) |
| Education level ≤3 school years | 89 (29.4) |
| Education level ≤6 school years | 266 (87.8) |
| Graffar social classification | |
| Graffar 1 | 2 (0.7) |
| Graffar 2 | 13 (4.3) |
| Graffar 3 | 106 (35.5) |
| Graffar 4 | 174 (58.2) |
| Graffar 5 | 4 (1.3) |
| Very low monthly income (<530Euros) | 197 (65.7) |
| Low monthly income (530–1060Euros) | 83 (27.4) |
| Mean smoking amount (pack/years) | 49.3±32.4 |
| mMRC grade ≥2 | 185 (61.1) |
| CAT score ≥10 | 152 (72.4) |
| Frequent ECOPD (≥2/previous year) | 115 (38.0) |
| Post-bronchodilator mean FEV1% | 53.2±19.7 |
| GOLD stage | |
| I | 30 (9.9) |
| II | 127 (41.9) |
| III | 106 (35.0) |
| IV | 40 (13.2) |
| Gold 2017 classification | |
| A | 70 (23.1) |
| B | 120 (39.6) |
| C | 7 (2.3) |
| D | 106 (35.0) |
Note: Data shown as mean±SD or n (%).
Abbreviations: mMRC, Medical Research Council Dyspnea Questionnaire; CAT, COPD Assessment Test; ECOPD, COPD exacerbations; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
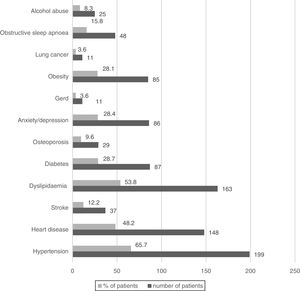
Wheezing was reported by 82.5% of patients, but only in 3.3% was it a frequent symptom. Chronic bronchitis (CB) was reported by 24.7% of patients (23.5% men and 33.9% women, p=0.085) and 32.3% of them were frequent exacerbators. The most prevalent comorbid conditions are presented in Figs. 1 and 2. Only 9.5% of patients presented no comorbid conditions, and 14.2%, 17.5%, 17.8%, 20.5% and again 20.5% present respectively 1, 2, 3, 4 and ≥5 comorbid conditions.
Eighty-two patients (27.4%) reported a previous diagnosis of asthma under the age of 40, usually in childhood. It was not possible to confirm the data in the hospital data base or on health data platform. 173 patients had undergone a pulmonary computed tomography (CT) scanning in the previous 6 years and bronchiectasis was found in 45 of them, being of cylindrical type and clinically unexpected in 31 patients. Long-term oxygen therapy (LTOT) had been prescribed to 45 patients and home non-invasive ventilation (NIV) to 24 patients (16 under LTOT+NIV). The particular characteristics of these patients are beyond the scope of the present study.
Relationship between different variablesTable 2 presents the relationship between different variables. Current smokers have a significantly lower mean age than ex-smokers or never-smokers (respectively 61.2, 67.2 and 72.5 years, p<0.001). We found no association between pack-years smoking history and GOLD stage (the average number of pack-year was 45.1, 44.6, 56.0 and 47.3 from GOLD 1 to 4, p=0.126). There was no significant association between GOLD 2017 stage and classification and hypertension, heart diseases, diabetes and dyslipidaemia. Obesity was less prevalent in GOLD 4 (23.3% in GOLD 1, 35.4 in 2, 28.3 in 3 and 7.5% in 4, p=0.007) and underweight in GOLD 1. When controlling for age, gender, monthly income, education level, smoking history, FEV1, chronic bronchitis or previous history of asthma (Table 3), only FEV1% and chronic bronchitis were associated with more symptomatic impact (CAT ≥10). When controlling the same variables (Table 3), only FEV1, chronic bronchitis and education level were associated with breathlessness (mMRC ≥2).
Relationship between different variables.
| Comparison by gender | Male gender | Female gender | p-value |
|---|---|---|---|
| Mean age | 67.4 | 67.6 | 0.886 |
| Mean FEV1% | 53 | 53.8 | 0.770 |
| CAT ≥10 | 68.5% | 88.1% | 0.007 |
| mMRC ≥2 | 59% | 72.1% | 0.400 |
| ECOPD ≥2 | 34.0 | 53.2% | 0.005 |
| Comparison by symptoms | CAT <10 | CAT ≥10 | p-value |
|---|---|---|---|
| Mean age | 67.9 | 64.7 | 0.035 |
| Mean FEV1% | 64.0 | 48.4 | 0.001 |
| ECOPD ≥2 | 10.3% | 46.1% | 0.001 |
| Comparison by dyspnoea | mMRC <2 | mMRC ≥2 | p-value |
|---|---|---|---|
| Mean age | 67.8 | 67.2 | 0.639 |
| Mean FEV1% | 63.5 | 46.6 | 0.001 |
| ECOPD ≥2 | 16.5 | 51.9 | 0.001 |
Note and abbreviations: Data shown as mean±SD or n (%); mMRC, Medical Research Council Dyspnea Questionnaire; CAT, COPD Assessment Test; ECOPD, COPD exacerbations; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Bold values indicate the statistical significance.
Binary logistic regression.
| Wald | df | Sig. | OR | 95% C.I.for OR | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Predictors of CAT | ||||||
| Smoking history | 1.409 | 1 | .235 | 2.167 | .604 | 7.769 |
| Gender | 3.770 | 1 | .052 | 4.414 | .986 | 19.757 |
| Age (≥65) | 2.241 | 1 | .134 | .546 | .247 | 1.206 |
| Monthly income | .009 | 1 | .924 | 1.040 | .468 | 2.312 |
| Education level | .031 | 1 | .859 | 1.098 | .392 | 3.071 |
| FEV1% | 18.775 | 1 | .000 | .957 | .937 | .976 |
| Asthma | .748 | 1 | .387 | 1.460 | .619 | 3.445 |
| CB | 5.382 | 1 | .020 | 3.974 | 1.239 | 12.746 |
| Constant | 4.554 | 1 | .033 | 9.497 | ||
| Predictors of mMRC | ||||||
| Smoking history | .000 | 1 | .989 | .994 | .437 | 2.261 |
| Gender | .525 | 1 | .469 | 1.376 | .580 | 3.261 |
| Age (≥65) | .649 | 1 | .420 | .775 | .416 | 1.442 |
| Monthly income | .786 | 1 | .375 | .765 | .423 | 1.384 |
| Education level | 5.092 | 1 | .024 | .461 | .235 | .903 |
| FEV1% | 32.916 | 1 | .000 | .955 | .940 | .970 |
| Asthma | .328 | 1 | .567 | 1.206 | .636 | 2.287 |
| CB | 7.014 | 1 | .008 | 2.679 | 1.292 | 5.555 |
| Constant | 21.587 | 1 | .000 | 31.181 | ||
Abbreviations: mMRC, Medical Research Council Dyspnoea Questionnaire; CAT, COPD Assessment Test; Asthma, previous history of asthma before 40th years; CB, chronic bronchitis.
Bold values indicate the statistical significance.
Patients reporting a previous diagnosis of asthma were younger (mean age=65.35/68.06 years, p=0.041), reported lower mean tobacco amount (46.35/58.32 pack-years, p=0.020) and were more symptomatic (CAT ≥10 in 82.3%/68.5%, p=0.029). However, there were no differences between the two groups related to gender (p=0.114), previous acute exacerbations (p=0.347), mean FEV1% (p=0.135) and distribution according to GOLD stage (p=0.637). CB was significantly related to GOLD classification (respectively 10.3, 27.8, 28.6 and 33.3% of GOLD A, B, C and D patients reported CB, p=0.011) but not to age, airflow limitation or GOLD stage.
Age and smoking history were not associated to an increased risk of exacerbation. Table 4 describes the relationship between ECOPD and different variables. Binary logistic regression indicates an odds ratio of 1.7 times more risk of ECOPD for very-low educational level (≤3 years of school) and 1.9 times more risk of exacerbations for lower income, when controlling for age and gender. The mean FEV1% of patients reporting 0, 1 or ≥2 acute exacerbations was respectively 59.2, 55.8 and 44.5, p<0.001. Higher airflow limitation, according to GOLD stage, was also associated with risk of acute exacerbation (from GOLD 1 to 4, 10.0%, 29.1%, 45.3% and 67.5% of patients reported ≥2 ECOPD, p<0.001). However, when controlling all the variables associated with frequent acute exacerbations, only CAT score and FEV1% had a statistical significant association with ECOPD (Table 5).
Relationship between ECOPD and other variables.
| ECOPD ≥2 | p-value | |
|---|---|---|
| Education level ≤3 years | 51.7 | |
| Education level >4 years | 32.2 | 0.001 |
| Monthly income <530Euros | 44.2 | |
| Monthly income ≥530Euros | 26.2 | 0.002 |
| Male gender | 34 | |
| Female gender | 53.3 | 0.005 |
| mMRC ≥2 | 51.9 | |
| mMRC <2 | 16.5 | 0.001 |
| CAT ≥10 | 46.1 | |
| CAT <10 | 10.3 | 0.001 |
Note and abbreviations: Data shown as %; mMRC, Medical Research Council Dyspnoea Questionnaire; CAT, COPD Assessment Test; ECOPD, COPD exacerbations.
Binary logistic regression – predictors of exacerbation (ECOPD).
| Wald | df | Sig. | OR | 95% C.I. for OR | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| mMRC grade | 1.348 | 1 | .246 | 1.650 | .709 | 3.842 |
| CAT score | 6.015 | 1 | .014 | 4.058 | 1.325 | 12.429 |
| FEV1% | 8.207 | 1 | .004 | .968 | .947 | .990 |
| Gender | 3.706 | 1 | .054 | 2.845 | .981 | 8.249 |
| Monthly income | .618 | 1 | .432 | .727 | .329 | 1.609 |
| Education level | 1.848 | 1 | .174 | .531 | .213 | 1.322 |
| Asthma | 1.966 | 1 | .161 | .580 | .271 | 1.242 |
| CB | .474 | 1 | .491 | 1.337 | .585 | 3.055 |
| Age (≥65) | 3.583 | 1 | .058 | 2.106 | .974 | 4.554 |
| Smoking history | 3.159 | 1 | .076 | .367 | .121 | 1.109 |
| Constant | .692 | 1 | .405 | 2.426 | ||
Abbreviations: mMRC, Medical Research Council Dyspnoea Questionnaire; CAT, COPD Assessment Test; Asthma, previous history of asthma before 40th years; CB, chronic bronchitis.
Bold values indicate the statistical significance.
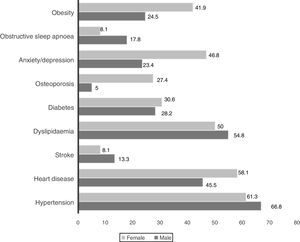
To the best of our knowledge this is the first study developed in a Portuguese population of COPD hospital outpatients fully describing their demographic, clinical and functional characteristics. Our data confirms COPD as a more prevalent condition in the elderly, in men, and people with low socioeconomic level. It also confirms previous evidence that a substantial proportion of patients were non-smokers, mainly women. There were four main findings in this study: (1) Women suffering from COPD smoked less, were more symptomatic and more frequent exacerbators. Obesity, osteoporosis and depression/anxiety were more common in the female gender. (2) Patients with a previous history of asthma were more symptomatic despite being younger and smoking less. (3) Predictors of ECOPD were gender, monthly income, education level, symptoms and airflow limitation. (4) FEV1 was significantly related both to symptoms and exacerbations.
In the present study, as expected, tobacco smoking was the most commonly identified exposure, but a significant proportion of patients (26%) was never-smokers who self-reported significant indoor exposure to household air pollution or occupational exposure to dust, gas or fumes relevant to COPD. COPD is known to be predominantly a disease of cigarette smokers. However, international variation in the prevalence of COPD cannot be explained solely by different rates of smoking in the community, and some authors estimated that at least one fourth of patients with COPD are never-smokers.11 A COPD prevalence of 9.2% was found in never-smokers in Lisbon region, but we have no information about the rate of never-smokers in COPD cases.12 In a recent analysis of data from 14 countries participating in the BOLD study, never-smokers comprised 23.3% of COPD patients classified as GOLD stage II+ and NHANES III data in US populations showed that about one-fourth of COPD cases occurred in non-smoking subjects.13 The analysis of BOLD data in Tunisia shows that non-smokers account for 45% of COPD cases,14 and a recent Korean study reported never-smokers accounting for 31.7% of all COPD patients.15 In the present study, never-smoking COPD patients were more symptomatic than patients with a smoking history, and contrasts with other published studies.16 This is an intriguing issue, and may partly be due to not having been considered as exclusion criteria in the present study, features that could suggest airway hyperreactivity. We found no association between smoking history and degree of airflow limitation. This also contrasts with previous studies.17
In our study, occupational exposure was self-reported and dichotomised as regularly-exposed and not-regularly exposed, because there was a significant lack of power in the information related to time and type of exposure.18 It was reported by 55.5% of patients, significantly overlapping with tobacco exposure. Occupational exposure as a COPD risk factor has long been recognised.19 The contribution of occupational exposure to the burden of COPD is estimated to be of 15%20,21 to 20%,13 but as much as 31% of COPD in never-smokers may be attributed to occupational exposure.22 Although population specific job exposure matrices (JEMs) perform better than the self-reported method in assessing the risk of developing occupational COPD,23 many epidemiological studies have relied on self-reported exposure,19 which can be subject to recall bias. Mineral dust exposure is a well-known cause of COPD, but a significant risk of COPD is associated to occupational exposure to biological dust,19 especially for women.
Indoor air pollution due to solid fuel combustion is an important risk factor for COPD,24 particularly in non-smoking women of middle and low-income countries.25 In this study, self-reported exposure to household air pollution from coal and biomass fuel combustion for cooking, both in the past and present, was reported by 63.8% of women. It was the most important exposure identified among women suffering from COPD. They were more symptomatic and more frequent exacerbators. Whether this represents a true gender characteristic or a greater tendency for women to report symptoms and exacerbations cannot be answered in the present study. Anxiety/depression, obesity and osteoporosis were more prevalent in the female gender. Some of this data is supported by previous studies.26 Being older was not related to airflow limitation, symptoms or acute exacerbations. These findings are in contrast with some other observational studies.27
ECOPD were defined according to GOLD, but also as requiring an unplanned medical visit. Therefore, our ECOPD definition fails to capture unreported and untreated minor or even moderate exacerbations. Probably unreported cases were less severe, self-limited and self-managed. Nevertheless, according to current literature, less than one third of exacerbations are estimated to be reported,28 but the unreported also have impact on patients’ health status. On the other hand, sudden respiratory symptoms due to comorbidities like congestive heart failure, pulmonary embolism or pneumonia can mimic ECOPD or even trigger a COPD acute exacerbation, leading to a real possibility of misclassification of some reported ECOPD. The clinical tools used in the present study were not calibrated to discriminate with high precision the causes of exacerbations or accurately distinguish ECOPD from other clinical situations. In our study, 38% of patients, more often women, were frequent exacerbators. As in other populations studied, low educational level and low monthly income were also associated with a history of previous acute exacerbation.29 The prognosis of COPD is closely related to the severity and frequency of acute exacerbations30: they influence the overall severity of the disease, impair quality of life, and worsen the pulmonary function and the underlying co-morbidities.28 Our study also failed to capture the aetiology and severity of COPD exacerbations. There is currently no consensual classification of exacerbation severity, and in one-third of cases the aetiology cannot be identified.31 However, different aetiologies and severity of acute exacerbations have different impact on health status, respiratory function and quality of life.
There was a positive association between airflow limitation and both symptoms and a history of frequent acute exacerbations. When controlling all variables associated with symptoms and exacerbations, only FEV1 was significantly related to mMRC, CAT and ECOPD. This is consistent with other published studies, and it is well-known that exacerbations are significantly associated with worsening lung function, and become more frequent and more severe as the severity of the underlying disease increases.
In the present study, 27.4% of COPD patients reported a previous diagnosis of asthma before 40, usually in childhood. Because of the possibility of confusion between COPD and asthma, in which dyspnoea is also a cardinal symptom, many studies on COPD exclude participants with a prior history of asthma.20 Because it is a well-known risk factor for COPD, we did not exclude those patients from the present study. They were younger, reported less tobacco consumption and more symptoms. As asthma and COPD are different conditions, features of both diseases can be present in the same patient. This is currently referred to as Asthma-COPD overlap (ACO). The definition, the clinical characteristics, the course and the phenotypes of ACO are still controversial.32 The diagnostic criteria changed during the period our study was designed and data collected and interpreted.33 Because neither the term nor the diagnostic criteria are universally accepted,34 and the Portuguese consensus on ACO was only recently published,35 ACO was not included for analysis.
COPD is widely recognised as a heterogeneous disorder with many systemic features and medical comorbidities occurring across the spectrum of disease severity.36 As in previous studies, our COPD patients present many different comorbidities, such as hypertension and heart diseases.1 The prevalence of diabetes, anxiety and depression was higher than in other studies.37 Gastroesophageal reflux disease (GERD) was present in only 3.6% of patients. Although we expected a higher prevalence, this is not surprising, because there are many different definitions of GERD based on different diagnostic models.
Significant and relevant differences in demographic and clinical characteristics and exposures relevant for COPD have been described in different countries. We recruited a convenience sample of COPD outpatients in the respiratory clinic of a single hospital, though middle-sized one, as the reference population. Nevertheless, the descriptive and analytic characteristics of most of the demographic variables and some clinical and functional ones show that the sample is representative of the studied population.38 As expected, descriptive and analytic characteristics of some variables differ from populations in other studies. This is due both to the fact that we included only outpatients recruited in a hospital setting, and because we studied a clinical sample.
ConclusionsOur data confirms COPD as a complex and heterogeneous disorder with significant morbidity due to the nature of symptoms, frequent comorbidities and exacerbations. Our data also confirms previous evidence that a substantial proportion of COPD patients, mainly women, are non-smokers. This calls attention to the need for COPD recognition among never-smokers, particularly women. COPD in female gender, in never-smokers and patients with a previous diagnosis of asthma presented some specific characteristics. Some patients’ characteristics are associated with frequent acute exacerbations. FEV1 was strongly related to both symptoms and acute exacerbations.
Author contributionsDuarte-de-Araújo conceived and developed the study, carried out acquisition and interpretation of data, wrote the first draft and collaborated in the final writing. Pedro Teixeira carried out the statistical analysis and contributed to the section on methods and results. Venceslau Hespanhol reviewed the final draft. Jaime Correia-de-Sousa reviewed all the drafts and collaborated in the final writing. All the authors approved the final manuscript.
Conflicts of interestThe authors have no conflict of interest to declare regarding the present study.