There is no doubt that the main risk factor for the development of chronic obstructive pulmonary disease (COPD) in developed countries is tobacco smoking,1 but there are other factors that may aggravate the course of COPD or may even be responsible for the persistence of bronchial inflammation and progression of the disease after quitting smoking. Among these factors, bronchial infection by potentially pathogenic microorganisms (PPMs) has generated great interest and its potential treatment has been included in guidelines of COPD treatment.2-4
The repeated isolation of PPMs, mainly Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis and Pseudomonas aeruginosa, from bronchial secretions in stable COPD (outside a period of exacerbation) has been associated with increased bronchial and systemic inflammation, increased respiratory symptoms, faster decline in lung function, frequent and severe exacerbations, poor quality of life, higher frequency of bronchiectasis, increased frequency of cardiovascular events, and even higher mortality.5-11 This clinical impact of the presence of PPMs in the airways of patients with stable COPD has generated the concept of chronic bronchial infection (CBI) in contrast to the usual term of bronchial colonization, that should be reserved for the presence of bacteria, usually commensal bacteria, without invading tissues or causing damage.10,12 However, the precise definition of CBI has been elusive; a group of experts have proposed that the isolation of the same PPM in at least three sputum samples separated by at least one month over one year could define CBI,12 but this definition still needs to be validated and globally accepted.
Existing evidence suggest that the concept of CBI should be differentiated from the single isolation of a PPM in sputum. There is evidence that CBI by P. aeruginosa in COPD patients is associated with a higher mortality8,13; however no significantly increased risk of death has been observed associated with a single isolation of this microorganism.13 This finding could have important therapeutic consequences because we should avoid the progression from a single isolation to established CBI; however definitive evidence of this progression in patients with COPD is still lacking. Nevertheless, the recognised impact of CBI highlights the importance of microbiological monitoring of respiratory samples (usually sputum) even in the stable phase of COPD, in particular in the most challenging patients with frequent exacerbations.4
Whatever the definition of CBI is, the repeated isolation of PPMs in sputum of patients with COPD is one of the main characteristics of the so-called “infective phenotype” of COPD, characterised by the production of coloured sputum, poor quality of life and frequent and severe exacerbations.14 The first consequence of the identification of this phenotype is that these patients must be examined by chest computed tomography to evaluate the presence and extent of bronchiectasis. There is evidence from prospective studies that frequent and severe exacerbations may lead to the development of bronchiectasis in COPD patients,11 which, in turn, closes the vicious circle because the presence of bronchiectasis is also a risk factor for the presence of CBI, especially by P. aeruginosa, more exacerbations and reduced survival.15,16
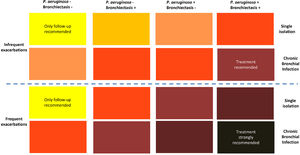
The second consequence is that due to the deleterious effect of CBI, treatment strategies should be applied to prevent lung damage.2,12,16 Unfortunately, there are no therapeutic trials for CBI specifically in COPD patients (with or without bronchiectasis), and the current strategies are based on the experience of treatment of CBI in bronchiectasis patients.17,18 Based on this experience, some recommendations have been published regarding the use of antibiotic treatment for CBI in COPD, including macrolides as immunomodulatory agents against neutrophilic inflammation, and systemic or inhaled antibiotics.6,12 These recommendations take into account four different factors: 1) the frequency of exacerbations, 2) the presence of bronchiectasis, 3) the isolation of P. aeruginosa, and 4) whether there is a single isolation of a PPM or a CBI. Recommendations for antibiotic treatment are depicted in colours in Fig. 1; the darker the color the stronger the recommendation for antibiotic treatment.6 Extreme cases would be COPD patients with frequent exacerbations, bronchiectasis, and CBI by P. aeruginosa, who must receive antibiotic treatment, according to the bronchiectasis guidelines,19,20 with the objective of eradicating, or at least reducing the bronchial bacterial load, if at all possible. The other end of the spectrum would be represented by a patient with infrequent exacerbations, without bronchiectasis and a single isolation of a PPM non P. aeruginosa, who would only require the usual follow-up, but no antibiotic treatment at this stage. Between these extremes, there is a whole range of intensities in the recommendations for antibiotic treatment, which must be individualized, mainly considering the presence of multiple and/or severe exacerbations as a possible consequence of the CBI (Fig. 1).6
Strength of the recommendation of antimicrobial treatment of CBI in COPD.
The darker the color, the stronger the recommendation of treatment; from yellow: only follow-up in COPD patients with a single isolation of a PPM, without frequent or severe exacerbations, no bronchiectasis, and no P. aeruginosa isolation; to dark gray: strong recommendation of treatment in COPD patients with CBI by P. aeruginosa and bronchiectasis with frequent or severe exacerbations.
‘International Journal of Chronic Obstructive Pulmonary Disease 2022 17 621–630′ Originally published by and used with permission from Dove Medical Press Ltd.’ (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Interestingly, there is a group of patients with COPD, who despite optimal inhaled bronchodilator and anti-inflammatory treatment, usually in the form of triple therapy (long-acting anticholinergic agent -LAMA-, long-acting beta-2 agonist -LABA- and inhaled corticosteroid -ICS-) still suffer from exacerbations.21 One reason for this is that triple therapy does not address the infectious component of exacerbations. These patients who still exacerbate despite optimal inhaled therapy must be investigated for the presence of bronchiectasis and CBI and be treated accordingly.12
The type of treatment for CBI in COPD is still controversial due to the lack of evidence; however, there is consensus among specialists that long-term macrolides can be a good option in CBI by PPMs other that P. aeruginosa, while inhaled antibiotics are preferred in cases of CBI by this pathogen.12,22 Some large observational studies in patients with COPD and bronchiectasis have observed significantly better outcomes with the use of long-term macrolides compared with ICS in terms of a reduction in moderate or severe exacerbations and even in improved survival.23 These results are probably related to the fact that the use of ICS may increase the risk of CBI by P. aeruginosa and pneumonia in COPD patients, in particular in patients with low blood eosinophil counts.24 As a consequence of the excessive use of ICS in patients with COPD,25 some important questions arise: What is the risk of ICS treatment in a COPD patient with CBI independently of the presence of bronchiectasis? Should these patients always be initially treated with macrolides? There are no clear responses, but these questions received the highest scores among 230 questions selected in an international consensus about research priorities in COPD for the next decade.26
In conclusion, the scientific community should be aware of the great importance of CBI in stable COPD and the urgent need for therapeutic studies with preventive antibiotics focused on preventing exacerbations, avoiding the development of irreversible bronchial damage or bronchiectasis and, in turn, improving the quality of life and the prognosis of our patients.
FundingThis manuscript was not funded.