Stone quarry workers account for about 30% of the tuberculosis (TB) cases1 in Penafiel and Marco de Canaveses, two municipalities in the northern region of Portugal that have the highest TB notification rates of the country.2
In high incidence areas, only a small number of reliable epidemiologically linked cases are identified using conventional contact investigations.3 Whole-genome sequencing (WGS) is increasingly used to study transmission dynamics.
We conducted a retrospective study including all notified cases of TB in stone quarry workers from the municipalities of Penafiel and Marco de Canaveses from 1 January 2012 to 31 December 2019. First, we analysed classical epidemiological data from the stone quarry workers with TB diagnosed during 2012–2014. Secondly, all the available strains of M. tuberculosis isolated from 2015 to 2019 were sent for WGS in the National Reference Laboratory using a single nucleotide polymorphisms (SNP)-based approach.4 Then we compared clustered and non-clustered cases using the Chi-squared test or Fisher's exact test.
According to local public health services’ records, 11 stone quarry workers with confirmed TB were notified between 2012 and 2014. Work-related exposure led to universal screening initiatives in workplaces: 135 co-workers of the same companies were screened. TB disease screening included chest-X ray and symptom questionnaire (96.3% adherence; one case found in 130 screened) and TB infection screening was performed through Tuberculin Skin Test (36.3% adherence; 30 cases found in 49 screened; two initiated preventive treatment). During 2016–2017, eight new cases were found among those previously screened individuals, i.e., 5.9% of the co-workers developed TB disease in a period of three years. The results of their screening were not screened (one), incompletely screened (four), not treated TB infection (two), previous negative screen (one). TB infection screening was not performed in more than 60% of the contacts, which may have contributed to the high proportion of screened workers that developed TB. However, we hypothesize whether this could reflect that transmission occurred in other settings besides workplaces (namely social or familiar).
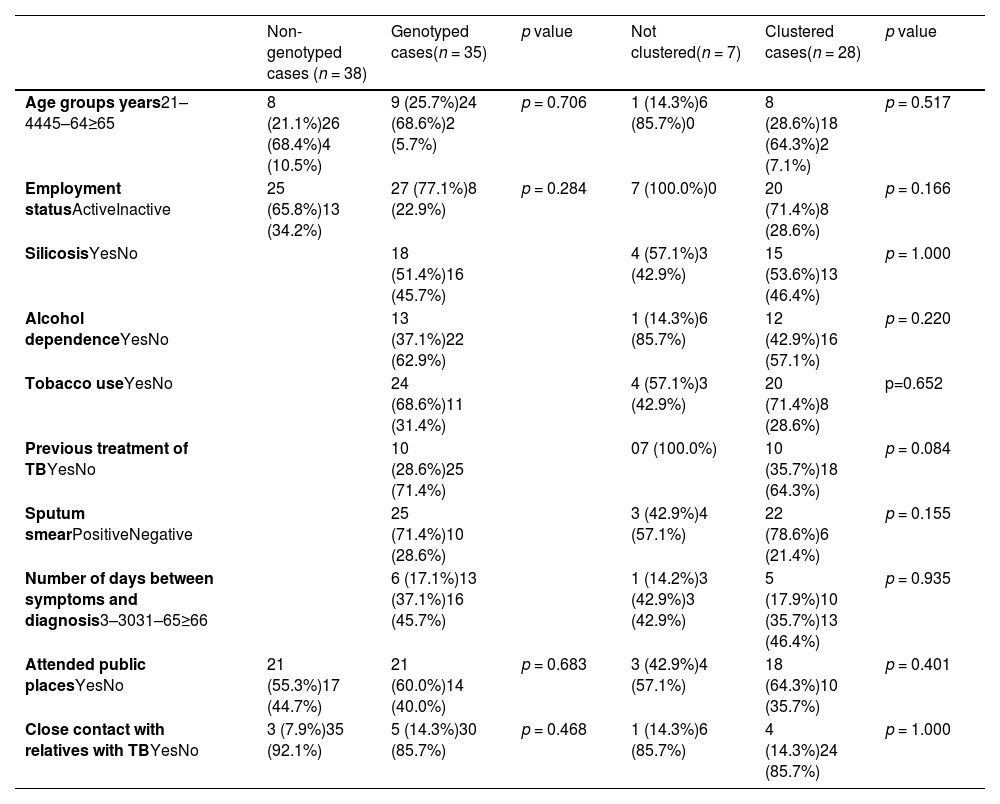
A total of 76 current or former stone quarry workers diagnosed with TB in the 2015–2019 period were found, i.e., 18.8% of the notified cases of TB in 2015–2019.2 Three of those were excluded (not confirmed TB). Of the 73 included cases, 35 (47.9%) had available specimens. Genotyped and non-genotyped cases of TB had similar characteristics regarding the considered risk factors (Table 1).
Characteristics of clustered and non-clustered TB cases.
All the cases were male, born in Portugal, with an average age of 50-years-old (median 51, interquartile range (IQR) 43–56), and had pulmonary TB. The main risk factors included tobacco use, silicosis and alcohol dependence (Table 1). No cases of HIV were found (six had not registered HIV status). Two of the genotyped TB strains were polyresistant to isoniazid and streptomycin, and 33 were susceptible to all first line drugs. The delay between onset of symptoms and diagnosis was on average of 78 days (median 63 days; IQR: 34.0–88.0).
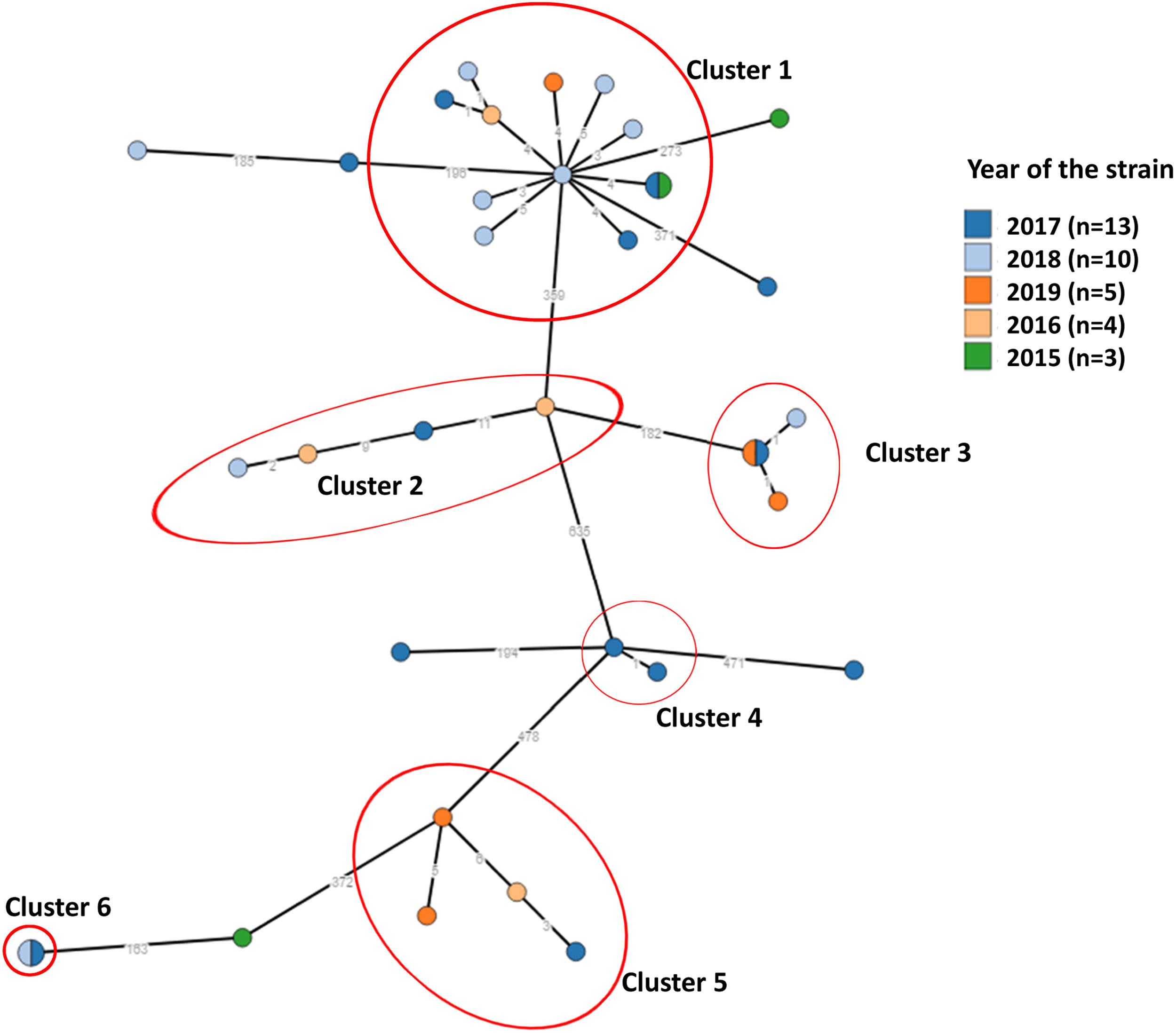
A high molecular diversity of M. tuberculosis was found (Fig. 1). Clusters included cases from 2015 to 2019, suggesting ongoing active transmission. As we did not genotype all the strains of M. tuberculosis from the community, we could be missing the remaining strains from other clusters.
Network of 35 Mycobacterium tuberculosis (MTB) isolates. Phylogeny of MTB isolates was determined based on a core single nucleotide polymorphisms (SNP) approach. Each node corresponds to a single or multiple isolates (i.e., nodes with slices). Nodes are coloured by year of strain isolation and genetic clusters of closely related isolates were defined whenever the strains had less than six SNP difference and are highlighted in red circles. Clades representing determined lineages from the global M. tuberculosis tree are indicated [cluster_1: lineage4.3.4.2 (100.0%, n = 12); cluster_2: lineage4.3.2 (100.0%, n = 4); cluster_3: lineage4.3.2 (100.0%, n = 3); cluster_4: lineage4.1.2.1 (100.0%, n = 2); cluster_5: lineage4.1.1.1 (100.0%, n = 4); cluster_6: lineage4.1.1.3 (100.0%, n = 2)].
Median (IQR) time between successive cases in clustered cases was 205 (95.5–308.3) days. Clustered cases were more prone to have alcohol dependence and smoking habits, which could be associated with attending coffee shops regularly (Table 1). Likewise, being professionally inactive, having a previous episode of TB and a positive sputum smear were also more common among clustered cases. As positive sputum smear indicates higher infectiousness, that is expected. In a study that analysed MIRU-VNTR molecular clustering data from 7458 patients, cases in large molecular clusters were also more likely to have multiple social risk factors.5
Using a logistic regression analysis, none of the transmission settings was a significant predictor of clusters but attending public places was the better predictor (OR 1.8, 95% CI 0.254–12.449). Social contacts in community public places such as coffee shops seem to contribute to the maintenance of ongoing active transmission of TB. In other study, workers that converted from IGRA negative to positive had no co-workers with active TB and were not identified as close contacts, suggesting they could have been infected in social settings.6
In our opinion, the high molecular diversity found in a small sample of stone quarry workers cases suggests a complex scenario of transmission between them and the high-risk communities in which they live and work. Stone quarry workers are not only more prone to transmit TB to other people of the community; they are also more susceptible to infection and re-infection by M. tuberculosis, given their common and multiple risk factors, especially in places where epidemiological links are difficult to establish. WGS could routinely contribute to identifying public places that are hotspots of TB transmission.



![Network of 35 Mycobacterium tuberculosis (MTB) isolates. Phylogeny of MTB isolates was determined based on a core single nucleotide polymorphisms (SNP) approach. Each node corresponds to a single or multiple isolates (i.e., nodes with slices). Nodes are coloured by year of strain isolation and genetic clusters of closely related isolates were defined whenever the strains had less than six SNP difference and are highlighted in red circles. Clades representing determined lineages from the global M. tuberculosis tree are indicated [cluster_1: lineage4.3.4.2 (100.0%, n = 12); cluster_2: lineage4.3.2 (100.0%, n = 4); cluster_3: lineage4.3.2 (100.0%, n = 3); cluster_4: lineage4.1.2.1 (100.0%, n = 2); cluster_5: lineage4.1.1.1 (100.0%, n = 4); cluster_6: lineage4.1.1.3 (100.0%, n = 2)]. Network of 35 Mycobacterium tuberculosis (MTB) isolates. Phylogeny of MTB isolates was determined based on a core single nucleotide polymorphisms (SNP) approach. Each node corresponds to a single or multiple isolates (i.e., nodes with slices). Nodes are coloured by year of strain isolation and genetic clusters of closely related isolates were defined whenever the strains had less than six SNP difference and are highlighted in red circles. Clades representing determined lineages from the global M. tuberculosis tree are indicated [cluster_1: lineage4.3.4.2 (100.0%, n = 12); cluster_2: lineage4.3.2 (100.0%, n = 4); cluster_3: lineage4.3.2 (100.0%, n = 3); cluster_4: lineage4.1.2.1 (100.0%, n = 2); cluster_5: lineage4.1.1.1 (100.0%, n = 4); cluster_6: lineage4.1.1.3 (100.0%, n = 2)].](https://static.elsevier.es/multimedia/25310437/0000003000000001/v1_202401030856/S2531043723000879/v1_202401030856/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)



