According to the literature, there is insufficient scientific data on the clinical impact, pre-post-analytical barriers, and health-care delivery, regarding the implementation of molecular tests for tuberculosis (TB) diagnosis recommended by World Health Organization (WHO) under field conditions in high burden countries.1
The molecular test Ultra assay (Xpert Ultra; Cepheid Inc., Sunnyvale, CA, USA) offers a lower limit of detection for Mycobacterium tuberculosis (MTB),2 being particularly important to detect paucibacillary TB presentations - extrapulmonary TB, TB/HIV coinfection and pediatric TB.5 Also, the test offers a semi-quantitative category of result named “trace”, which is considered a concern.
We aimed to evaluate the performance of Ultra under routine conditions in respiratory specimens tested at Thorax Diseases Institute - Federal University of Rio de Janeiro, Brazil. A diagnostic test study using secondary data from the Mycobacterial Laboratory included all spontaneous sputum (SS), induced sputum (IS) and bronchoalveolar lavage (BAL) submitted to Ultra, smear microscopy and automatized culture, between December 2019 and December 2020 (Ethics Committee approval #01561018.3.0000.5257). Tests with indeterminate results, contaminated cultures, non-tuberculosis mycobacteria identification and those performed during patient's follow-up were excluded.
Cases were classified as Confirmed TB (MTB identification on culture), Non-confirmed TB (clinical/radiological diagnosis, treated and cured) or Not-TB (other diagnosis concluded). Data for HIV-status were performed searching on https://laudo.aids.gov.br/login.
Medcalc® Statistical Software (MedCalc Software Ltd, Ostend, Belgium) was used for statistical analysis.
722 specimens (330 BAL, 260 SS and 132 IS) were analyzed, with 59/105 known HIV status being positive. Ultra's overall diagnostic sensitivity was 93.5% with a specificity of 91.2%. In smear positive samples, Ultra was 100% sensitive versus 87.6% in smear negative.
For different pulmonary specimens, Ultra was more accurate on BAL (93.6%), followed by IS and SS [Accuracy (Acc) 90.9% and 89.6%, respectively] (Table 1).
Ultra performance according to the different respiratory specimens.
Legend: TB – Tuberculosis; MTB – Mycobacterium tuberculosis; Se – Sensitivity; Sp – Specificity; PPV – Positive predictive value; NPV – Negative predictive value; PLR – Positive likelihood ratio; NLR – Negative likelihood ratio; Acc – Accuracy
The fifty false-positive Ultra results included 13 previous TB cases. In 10/154 TB cases, Ultra lead to false-negative results, and in all of them mycobacterial cultures identified MTB growth.
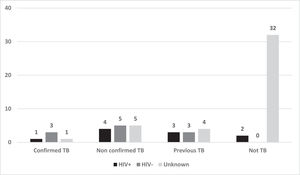
63/722 respiratory specimens presented trace results, five (7.9%) with confirmed TB and 14 (22.2%) with non-confirmed TB. 10/44 cases classified as not-TB had previous TB (Fig. 1). Therefore, Ultra was 93.5% sensitive and 91.2% specific. If trace results were considered as MTB not detected, sensitivity would fall to 81.1% with 98.9% of specificity. Whether the previous history was taken into consideration for classification of trace as MTB not detected and attributed to DNA fragments of non-viable bacilli, sensitivity and specificity would be both 93.5%.
To the best of our knowledge, this is the first study under routine conditions and in a scenario of high prevalence of TB, as Brazil, that analyzes the performance of Ultra in such a large sample, which turn this letter innovative and informative.
Recently, Xpert® MTB/RIF showed a good performance in different pulmonary specimens, with emphasis in IS (Acc=97%).3 In the present study, Ultra on IS had a sensitivity of 96.5% and a lower specificity of 89.3%, which is compatible with what the new version proposes. Considering the differences between our sample and Zar's et al,4 which addresses a pediatric sample, Ultra also presented a better performance on IS when compared to nasopharyngeal aspirate. Despite our results showing the greater accuracy on BAL, the performance of Ultra on IS deserves to be highlighted, considering the costs and safety of the method, which can be substituted for BAL for TB diagnosis.
For Mazzola and colleagues,5 Ultra's proportion of true-positive results exceeds the false-positives: the latter being avoidable in scenarios with a low pretest probability. In our casuistry, considering only the false-positive results attributed to previous TB history, 70.6% presented trace-results.
Independently of HIV-status, trace category increased case detection by 2.1% without significant loss of specificity in the study of Andama et al.6 In our research, if trace-results were considered negative in all cases, sensitivity would fall to 81.1%, with a higher specificity (98.9%). The other way, 44 cases would be false-positive, ten with previous TB. Therefore, if the history of previous pulmonary TB had been evaluated, and Ultra trace result considered as MTB not detected in this situation, we would find a balance of both parameters (93.5%).
In Kendall's7 publication, Ultra´s trace results increased overtreatment by more than 50%, nonetheless it avoided 50% of deaths. Therefore, the interpretation of trace results requires a balance between case detection and overtreatment as established by Global Laboratory Initiative of the Stop TB partnership.
Our limitations were mostly attributed to diagnostic test studies conducted in routine conditions. We could not analyze Ultra results according to HIV-status in all specimens and we didn't have access to all previous TB history.
In conclusion, our study, conducted in a country with a high burden for TB, confirmed Ultra's good overall performance for pulmonary TB diagnosis. Other studies conducted in different scenarios are needed to confirm the correct interpretation of trace results in this specific tuberculosis presentation.
Authors contributionAPS and FCQM: conception and design of the study; APS, GD, JGR, MCFFA; FMR: acquisition of data; APS: analysis and interpretation of data, drafting the article; FM, AK and DRS: revision of the paper for important intellectual content and final approval of the version to be submitted.