Despite current medical management, exertional breathlessness is commonly experienced by adults with severe asthma limiting their exercise tolerance. A cardiopulmonary exercise test may help identify the reasons for these symptoms to guide appropriate management and evaluate new interventions. For instance, exhalation can be interrupted by the next inspiration resulting in an increased end expiratory lung volume. Increasing end expiratory lung volume as ventilation increases is defined as dynamic hyperinflation.1 Although there are reports of dynamic hyperinflation in asthma,2-4 the frequency, severity and impact by exercise platform is unknown.
We aimed to assess: 1) the presence and magnitude of dynamic hyperinflation, and contribution to exercise intolerance in patients with severe asthma compared to healthy individuals; 2) whether dynamic hyperinflation was affected by the presence of exercise-induced bronchoconstriction and fixed airflow obstruction, or exercise modality. Some of the results of these studies have been previously reported in the form of an abstract.5
This study was a prospective (trial registration # ISRCTN96143888) cross-sectional study and part of a larger study investigating the feasibility of asthma-tailored pulmonary rehabilitation.6 Adults with severe asthma (Agroup) and MRC Dyspnoea ≥ 2, under the care of asthma specialists in a tertiary centre multi-disciplinary service were recruited at Glenfield Hospital, Leicester, UK. Fixed airflow obstruction was defined as an FEV1/FVC less than the lower limit of normal despite medical management. Exclusion criteria were >10 pack-year smoking history with the presence of fixed airflow obstruction. Age, sex matched, self-reported healthy individuals with no known co-morbid conditions and <10 pack-year smoking history were recruited as controls (Cgroup). The study was approved by the National Research Ethics Service Committee of the East Midlands (Ref#127552) and written informed consent was obtained before participation.
All Agroup performed spirometry, incremental treadmill (‘treadmill’) and cycle (‘cycle’) exercise tests, in random order, with bidirectional volume measures and expiratory gas analysis. Spirometry was repeated during recovery to assess exercise-induced bronchoconstriction.7 The Cgroup performed spirometry and the treadmill test only.
During all exercise tests, inspiratory capacity manoeuvres were performed at rest, during warm up and every two minutes during exercise. Assuming the participant inspired to total lung capacity (TLC), inspiratory capacity was calculated as the difference in TLC and the average of the last five end expiratory lung volume values (end expiratory lung volume = TLC – inspiratory capacity) observed before the participant was prompted to inhale to TLC.8 The presence and magnitude of dynamic hyperinflation was assessed through the linear regression of end expiratory lung volume (ml) as a function of minute ventilation (V˙E) with units ml·(L·min−1)−1.1,8 Using the average tidal volume (Vt) that preceded the prompt, end inspiratory lung volume = end expiratory lung volume + Vt and inspiratory reserve volume = TLC – end inspiratory lung volume were calculated. Maximal voluntary ventilation (MVV) was estimated FEV1 x 40 and ventilatory limitation was defined as V˙Epk >80%MVVpred.
Fifty-five patients with severe asthma and 30 controls were recruited (Table E1). Peak oxygen uptake (see Table E2) was significantly lower in Agroup compared to Cgroup (1971 ml·min−1 versus 2471 ml·min−1 respectively, p<0.001). A greater proportion of Agroup demonstrated a ventilatory limitation compared to Cgroup (49% versus 23% respectively, p<0.05).
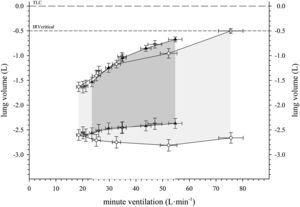
Shown in Fig. 1, end expiratory lung volume increased during treadmill exercise in Agroup (9 [23] ml·(L·min−1)−1) whereas it did not increase in the Cgroup (−1 [8] ml·(L·min−1)−1). The difference between groups was significant (difference = 10 [1 to 19] ml·(L·min−1)−1). In the Agroup there was no significant difference in dynamic hyperinflation during treadmill exercise compared to cycle exercise (difference = −4 [−9 to 2] ml·(L·min−1)−1).
Operational lung volumes plotted against ventilation on the incremental treadmill test in patients with severe asthma (black triangles) and healthy individuals (white circles). Tidal volume (greyed area) is bounded by the EELV (lower boundary) and EILV (upper boundary) for each group. Patients with severe asthma demonstrate DH whereas healthy individuals demonstrate the absence of DH (slope of EELV ≅ 0). Operational lung volumes in patients with severe asthma on the incremental cycle test are also shown (grey triangles). The error bars represent standard error. DH: dynamic hyperinflation, TLC: total lung volume, IRV: inspiratory reserve volume, EILV: end inspiratory lung volume, EELV: end expiratory lung volume, TLC: total lung capacity, IRV: inspiratory reserve volume.
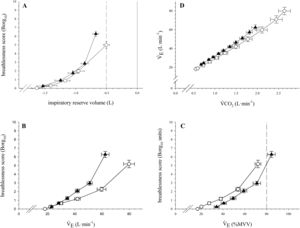
In contrast to the Cgroup, the Agroup demonstrated an inflection of increased breathlessness before the critical inspiratory reserve volume (Fig. 2A). The slope of the relationship between V˙E and V˙CO2 (Fig. 2D) was not different between Agroup and controls. However, Agroup indicated more breathlessness as assessed by Borg scale9 per absolute V˙E than Cgroup (Fig. 2B) but less of an effect when V˙E was expressed as percent predicted maximum voluntary ventilation (Fig. 2C).
A comparison of symptom response between patients with severe asthma (triangles) and healthy individuals (circles) on an incremental treadmill test including: breathlessness response as a function of inspiratory reserve volume (panel A) as well as a function of ventilation in absolute (panel B) and relative terms (panel C). The relationship of V˙E and V˙CO2 ventilation is shown in panel D. The error bars represent standard error.
In subgroup analyses, there was no significant difference in dynamic hyperinflation between: 1) Agroup with and without fixed airflow obstruction (14 [37] ml·(L·min−1)−1 versus 5 [9] ml·(L·min−1)−1, respectively, p = 0.17); 2) Agroup with and without exercise-induced bronchoconstriction (12 [43] ml·(L·min−1)−1 versus 9 [10] ml·(L·min−1)−1, respectively, p = 0.72).
We observed patients with severe asthma develop mild dynamic hyperinflation during incremental exercise whereas healthy controls maintained their end expiratory lung volume with increasing ventilation. In asthma, the dynamic hyperinflation was not affected by the exercise modality, exercise-induced bronchoconstriction or fixed airflow obstruction indicating evaluation of a physiological deficit not reflected by other physiological tests.
In the presence of mild dynamic hyperinflation, Agroup limited Vt relatively early in the exercise and did not increase end inspiratory lung volume to achieve a critically low inspiratory reserve volume, likely to avoid mechanical and sensory consequences associated with expanding their lungs close to TLC. Under these circumstances they were obliged to increase their respiratory rate to increase minute ventilation. Their breathlessness rose sharply during exercise which may be associated with reaching their flow related breathing capacity and less to do with the mechanical consequences of volume expansion close to TLC. In contrast, the majority of healthy individuals reached their critical inspiratory reserve volume. However, perceived breathlessness was significantly higher in patients with severe asthma compared to healthy individuals at maximal exertion (see Table E1).
The magnitude of dynamic hyperinflation in this study was assessed through the slope of regression line between end expiratory lung volume against ventilation with the advantage that test duration, power or ventilation achieved do not affect the assessment of dynamic hyperinflation in contrast to pre-post measures only.1 The magnitude of dynamic hyperinflation in patients with severe asthma in our study is similar to that in patients with mild COPD reported by O'Donnell et al.10 We did not observe a difference in dynamic hyperinflation between treadmill and cycle exercise demonstrating that the primary determinant of the end expiratory lung volume is the magnitude of ventilation. It has been suggested that dynamic hyperinflation would be greater during walking because intercostal respiratory and abdominal muscles stabilize the trunk and compromise expiration11 but our observations suggest this influence in inconsequential. We observed similar magnitude of dynamic hyperinflation in adults with severe asthma with and without exercise induced bronchoconstriction in contrast with a previous report3 of younger patients with mild asthma and non-fixed airflow obstruction.
The underlying mechanisms of dynamic hyperinflation observed in adults with severe asthma are likely to be different to those driving dynamic hyperinflation in COPD and deserve further study. Furthermore, the resultant ventilatory limitation to exercise may translate to adults with severe asthma reducing their physical activity in daily life as dynamic hyperinflation has been observed during lower exercise challenges such as the six-minute walk test.11 Whether optimal bronchodilation can ameliorate the dynamic hyperinflation remains to be determined.
ConclusionsThis study identifies dynamic hyperinflation as an important quantifiable consequence of severe asthma contributing to exercise limitation. In addition to the mechanical constraint, our data suggest that some patients with severe asthma may have an altered perception of breathlessness. The underpinning pathophysiology requires further investigation, but both phenomena are potential treatable traits.
Author contributionsRE contributed to the conception and design, analysis and interpretation of data, drafting and revising the manuscript critically for important intellectual content. SM contributed to the design, analysis and interpretation of data, drafting and revising the manuscript critically for important intellectual content. TD contributed to the conception, analysis and interpretation of data; drafting and revising the manuscript critically for important intellectual content. RHG, PB and SS contributed to the interpretation of data; revising the manuscript critically for important intellectual content. All co-authors contributed and approved the final manuscript. RE is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FundingThis work was funded by a Research for Patient Benefit grant of the National Institute for Health Research (NIHR) (grant no. PB-PG-0712-28063). Dr. Rachael Evans held a National Institute for Health Research (NIHR) clinician scientist fellowship CS-2016-16-020. This research was supported by the NIHR Leicester Biomedical Research Centre. The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
We would also like to thank all the members of the NIHR Leicester Biomedical Research Centre–Respiratory who supported Dr Sally Majd during her PhD studies. We are very grateful to all the patients who gave their time and effort to contribute to the study. We acknowledge and thank Dr Roger Goldstein and the Respiratory Diagnostics and Evaluation Services, West Park Healthcare Centre, Toronto, Canada for supporting the collaboration with Mr Thomas Dolmage.