Infectious diseases are one of the principle causes of morbidity, mortality and drain on health resources worldwide. In recent years there has been an increase in the impact of respiratory infections, particularly in the Portuguese population. It is for this reason that the Portuguese Respiratory Society has presented a series of recommendations for the prevention of respiratory infections in adults. These recommendations include both general measures and vaccinations for flu and pneumococcal pneumonia.
As infeções respiratórias são uma das principais causas de morbilidade, mortalidade e consumo de recursos de saúde a nível global. Nos últimos anos tem-se assistido a um crescente impacto das infeções respiratórias, nomeadamente na população portuguesa. Assim, a Sociedade Portuguesa de Pneumologia apresenta um conjunto de recomendações para a prevenção das infeções respiratórias no adulto. Estas recomendações englobam medidas gerais e de vacinação antigripal e antipneumocócica.
Respiratory infections are among the principal causes of morbidity, mortality and of demands on health resources at a global level.1 Apart from the direct and indirect costs, what is of major concern is the associated high consumption of antimicrobial drugs and the consequent increased growth in resistance to this class of medicines, which could affect the use of some types of antibiotics in the near future.
In continental Portugal, recently published data relating to the period of 2000–2009 document the significant impact of respiratory infections and, in particular, of pneumonias. In this period hospital admissions for Community Acquired Pneumonia (CAP) represent 3.7% of the total number of adult hospital admission for all causes in National Health Service institutions.2 In the age groups ≥50 and ≥65, hospitalization for CAP represents 5.5% and 7.0% of total admissions respectively.2
Given this national situation, there is a general consensus about the necessity for a rapid implementation of measures to prevent respiratory infections in adults.
These preventive measures against respiratory infection cover general measures and specific measures: vaccination against flu and antipneumococcal.
General measuresIn the general measures, related to host defences, the following are recommended3:
- -
smoking cessation;
- -
control of chronic illnesses (diabetes mellitus, COPD, congestive heart failure, chronic renal failure, chronic liver disease, HIV/AIDS infection, etc.);
- -
judicious use of immunosuppressive therapies (including corticosteroids);
- -
alcohol counselling (including acute intoxication and chronic alcoholism);
- -
advice about dealing with cases of drug addiction;
- -
adequate nutritional status;
- -
gamma globulin IV immunotherapy in selected patients (IgG deficiency, multiple myeloma, chronic lymphocytic leukaemia, transplant patients).
This endorses the Directorate-General of Health recommendation for vaccination against seasonal flu for the current season.4 Health professionals and other professionals involved in front-line health care are a priority group for vaccination, because of the increased risk of contracting the illness and transmitting it to their families and their patients. Setting an example and the counselling about vaccination given by health professionals represent one of the principle success factors in keeping to vaccination targets.
Pneumococcal vaccinationIn Portugal two pneumococcal vaccines are available for adults aged 18 and above, a pneumococcal polysaccharide vaccine 23-valente [(Pneumo 23®) VPP23] with 23 serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F) and a pneumococcal conjugate vaccine 13-valente [(Prevenar 13®) VPC13] with13 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F). Both vaccines are intramuscular and VPP23 can also be administered subcutaneously.5,6
For adults, vaccines are indicated for the prevention of invasive pneumococcal diseases by the serotypes included in the vaccine. The bacteraemia secondary to pneumonia is the main manifestation of invasive pneumococcal disease in adults, representing about 75% of cases of invasive disease in the adult population7 and more than 80% in those ≥65.8
At the present time, we are waiting for the results of studies about the efficacy of VPC13 in the prevention of pneumonia in adults.
Epidemiological surveillance programmes on invasive pneumococcal disease and its serotypes at a national level are crucial for the evaluation of the effectiveness of anti-pneumococcal vaccines and recommendations for their utilization. According to the latest data available at a national level, relating to the period 2006–2008, from a total of 1100 isolates of Streptococcus pneumoniae in adults with an invasive pneumococcal disease, 68% of the serotypes identified were included in VPC13 and 84% in VPP23.9
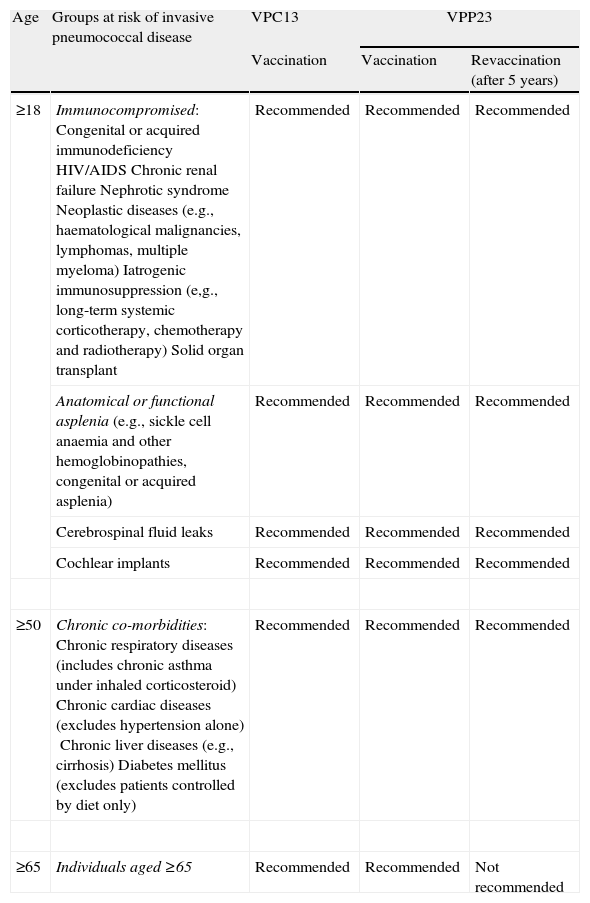
Table 1 presents recommendations for antipneumococcal vaccinations according to the risk of invasive pneumococcal disease.7,8,10–13
Recommendations for adult antipneumococcal vaccination.
| Age | Groups at risk of invasive pneumococcal disease | VPC13 | VPP23 | |
| Vaccination | Vaccination | Revaccination (after 5 years) | ||
| ≥18 | Immunocompromised:Congenital or acquired immunodeficiencyHIV/AIDSChronic renal failureNephrotic syndromeNeoplastic diseases (e.g., haematological malignancies, lymphomas, multiple myeloma)Iatrogenic immunosuppression (e,g., long-term systemic corticotherapy, chemotherapy and radiotherapy)Solid organ transplant | Recommended | Recommended | Recommended |
| Anatomical or functional asplenia(e.g., sickle cell anaemia and other hemoglobinopathies, congenital or acquired asplenia) | Recommended | Recommended | Recommended | |
| Cerebrospinal fluid leaks | Recommended | Recommended | Recommended | |
| Cochlear implants | Recommended | Recommended | Recommended | |
| ≥50 | Chronic co-morbidities:Chronic respiratory diseases (includes chronic asthma under inhaled corticosteroid)Chronic cardiac diseases (excludes hypertension alone)Chronic liver diseases (e.g., cirrhosis)Diabetes mellitus (excludes patients controlled by diet only) | Recommended | Recommended | Recommended |
| ≥65 | Individuals aged ≥65 | Recommended | Recommended | Not recommended |
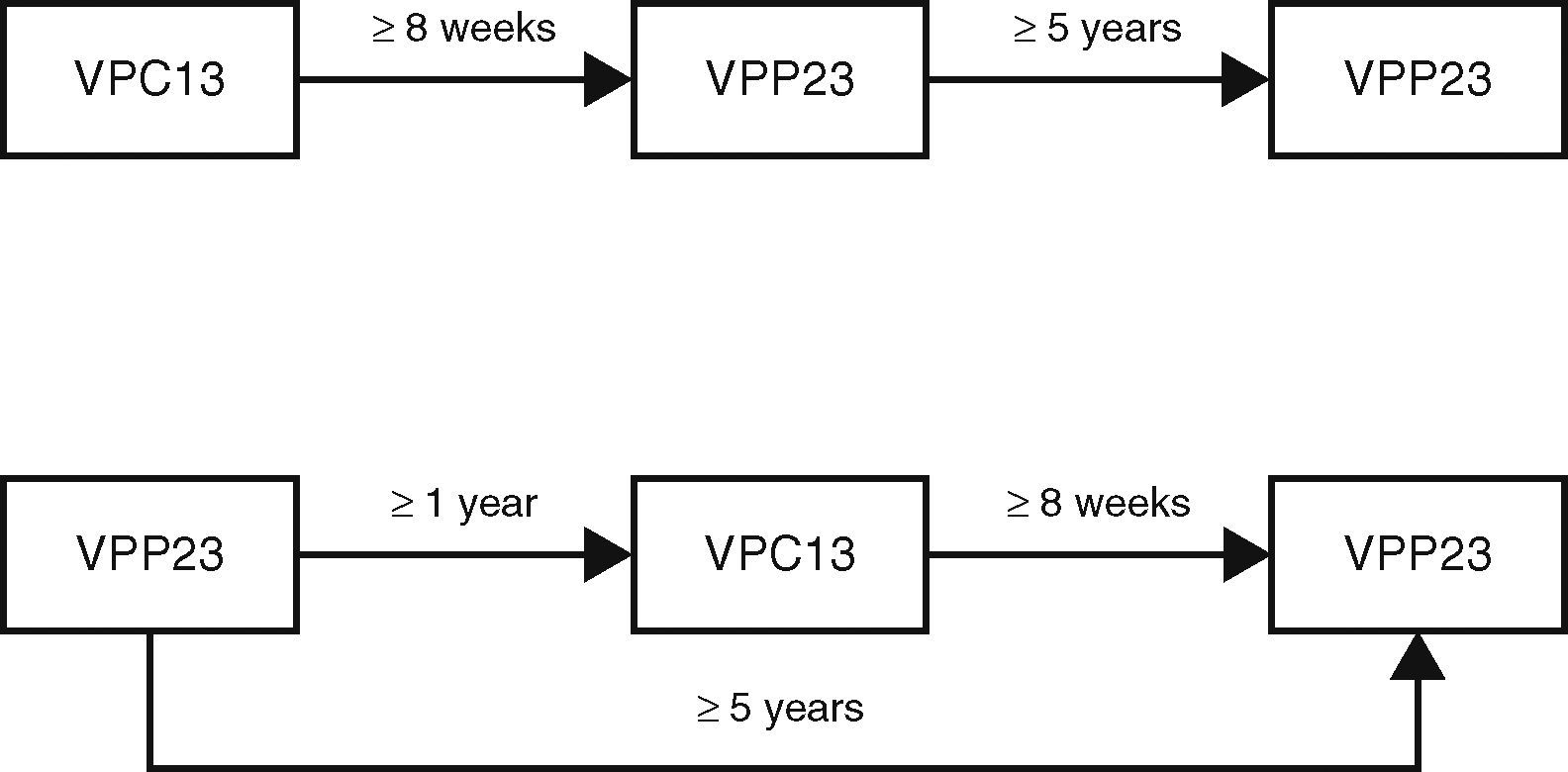
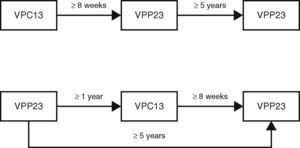
In adults who are indicated for antipneumococcal vaccination, the following dosing schedule is suggested10:
- -
adults who have not previously been vaccinated should first receive VPC13 followed by a dose of VPP23 at least 8 weeks later;
- -
adults who have already been vaccinated with VPP23 should only receive VPC13 at least one year after the last vaccination of VPP23;
- -
for adults with indication for revaccination with VPP23, the second dose must only be given at least 5 years after the first dose of VPP23 and at least 8 weeks after administration of VPC13.
Revaccination with VPC13 is not recommended and in the case of VPP23, revaccination should only be done once (Fig. 1).
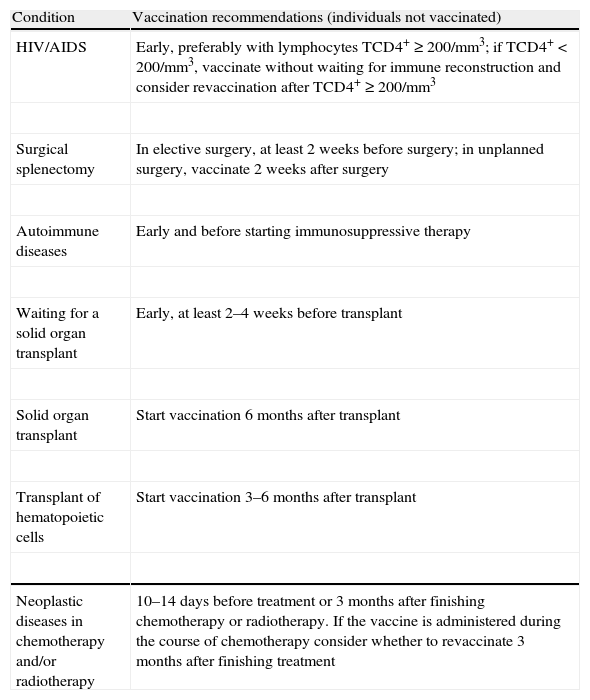
Pneumococcal vaccines should be administered at the most propitious time for the immune system to respond; Table 2 presents an optimization calendar of vaccinations for certain clinical conditions (adapted13).
Recommendations for antipneumococcal vaccination in specific circumstances (adapted from1,13).
| Condition | Vaccination recommendations (individuals not vaccinated) |
| HIV/AIDS | Early, preferably with lymphocytes TCD4+≥200/mm3; if TCD4+<200/mm3, vaccinate without waiting for immune reconstruction and consider revaccination after TCD4+≥200/mm3 |
| Surgical splenectomy | In elective surgery, at least 2 weeks before surgery; in unplanned surgery, vaccinate 2 weeks after surgery |
| Autoimmune diseases | Early and before starting immunosuppressive therapy |
| Waiting for a solid organ transplant | Early, at least 2–4 weeks before transplant |
| Solid organ transplant | Start vaccination 6 months after transplant |
| Transplant of hematopoietic cells | Start vaccination 3–6 months after transplant |
| Neoplastic diseases in chemotherapy and/or radiotherapy | 10–14 days before treatment or 3 months after finishing chemotherapy or radiotherapy. If the vaccine is administered during the course of chemotherapy consider whether to revaccinate 3 months after finishing treatment |
The influenza and pneumococcal vaccines can be given at the same time, preferably in different arms.
ConclusionsThe recommendations presented in this document should be subject to clinical judgement in relation to the individual cases.
This document will be subject to periodic revisions in order to include the scientific evidence of future studies and knowledge about other measures and vaccines like, for example, the whooping cough which is available and recommended in some European countries.14,15