Amyloidosis results from proteins being deposited as insoluble β-pleated sheets and disrupting organ function. Each precursor protein induces a separate spectrum of organ involvement, and different disease manifestations within the lung. Although autopsy findings often demonstrate amyloid deposits in various compartments of the lung, few are manifested pathologically. Amyloid lung nodules with positron emission tomography (PET) uptake are rare. We describe a rare case where PET uptake was detected in a pulmonary amyloid nodule. To our knowledge there are six previously reported cases in the English literature.
This review also focuses on amyloid derived from immunoglobulin light-chain protein (AL disease), which most frequently involves the lung in both systemic and localized forms of the disease. Manifestations of AL-related lung disease range from nodules identified on incidental chest films to diffuse alveolar and septal deposition mimicking malignancy and or diffuse alveolar damage.
A amiloidose é causada por proteínas depositadas como lâminas β insolúveis e que interferem com o funcionamento dos órgãos. Cada proteína precursora induz um espectro diferenciado de envolvimento dos órgãos, e diferentes manifestações de doença no pulmão. Apesar dos achados de autópsias revelarem frequentemente depósitos de amilóide em vários compartimentos do pulmão, poucos se manifestam patologicamente. A captação de nódulos de amilóide do pulmão na tomografia de emissão de positrões (PET) é rara. Descrevemos um caso raro em que foram detectados nódulos de amiloidose pulmonar através do PET. Que seja do nosso conhecimento, existem seis casos registados anteriormente na literatura Inglesa.
Esta análise também se centra na amiloidose derivada da proteína de cadeia leve de imunoglobulina (doença de AL – Amiloidose primária), que frequentemente envolve o pulmão tanto em formas localizadas como sistémicas da doença. As manifestações da doença pulmonar relacionadas com a AL variam desde nódulos identificados em incidências de raio-X do tórax até à deposição septal, simulando malignidade, e/ou em danos difusos alveolares.
Amyloidosis results from over-expression of specific proteins culminating in the extracellular deposition of insoluble β-pleated sheets of fibers. Initially identified as carbohydrate by Virchow1 in 1854, amyloid deposits are composed of linear arrays of subunit proteins in glucose-aminoglycans and serum amyloid P (SAP).
Amyloid deposits occur in systemic and organ-limited forms. In systemic amyloidosis, the composition of subunit proteins in the β-pleated sheets dictates the pattern of organ involvement and disease prognosis. Identifying the type of amyloidosis strongly impacts the diagnostic differential of radiographic abnormalities.
Classification of systemic amyloidosis is based on the different subunit proteins, which define organ involvement and disease manifestations. There are four major categories of systemic amyloidosis (“A” for amyloid and a letter indicating the protein involved): (1) primary or immunoglobulin light-chain (AL) disease, (2) secondary or amyloid protein A (AA) disease, (3) hereditary or mutant transthyretin (ATTR) disease, and (4) dialysis-associated or β2-microglobulin (β2M) disease. Other less prevalent forms of amyloid disease exist, locally affecting the pancreas (islet amyloid polypeptide, AIAPP) and brain (β-amyloid precursor protein, Aβ), or genetically transmitted systemic disease (apolipoprotein AI, gelsolin, fibrinogen, lysozyme precursor proteins). Pulmonary manifestations mainly occur from AL disease, this may be local or systemic depending upon the expression of monoclonal protein either just locally in the lung or in the serum/urine. Amyloid lung nodules with PET uptake are rare. We describe a rare case where positron emission tomography (PET) uptake was noticed in pulmonary amyloid nodules. To our knowledge there are six reported cases in the English literature to date2–7 (Table 1).
PET positive cases with amyloid described in the literature.
| Paper | Year of publication | Age/gender | Etiology of further workup | Further intervention |
| 1. Currie et al. | 2005 | 73 female | r/o ca | Video-assisted thorascopic wedge biopsy |
| 2. Ollenberger et al. | 2004 | 85 male | r/o ca | Upper lobe segmentectomy |
| 3. Umeda et al. | 2007 | 60 female | r/o ca | Video-assisted thorascopic biopsy |
| 4. Kung et al. | 2003 | 68 female | r/o ca | Lung lobectomy |
| 5. Yadav et al. | 2006 | 55 male | r/o ca | Wedge excision |
| 6. Grubstein et al. | 2005 | 59 female | na | na-comment: described 2 other cases of lymphoma |
r/o: rule out, na: not available.
A 68-year-old male with a past medical history significant for hypertension and osteoarthritis presented with bilateral knee pain worse for the last few months. He was a non-smoker, denied any abuse of alcohol or any other illicit substances. He had no known allergies and his past surgical history was only significant for an appendicectomy several years ago.
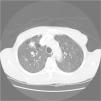
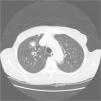
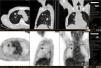
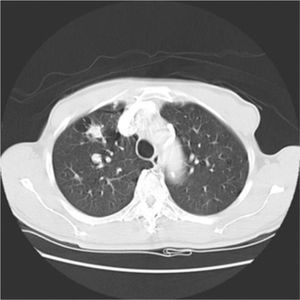
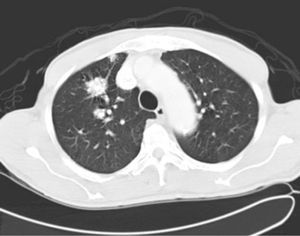
His general physical and systemic examination except for knee crepitus in both knees was otherwise normal. His laboratory examination including a complete blood count, basic chemistry and liver function tests including a clotting profile was normal. No abnormality was seen on an electrocardiogram and pulmonary function tests. On routine investigation his chest X-ray revealed a pulmonary nodule in the right upper lobe. His CT scan of the chest (see Figs. 1 and 2) showed pulmonary nodule 2cm×2cm in size.
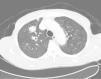
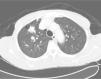
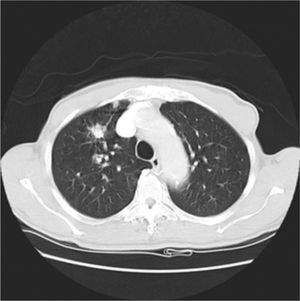
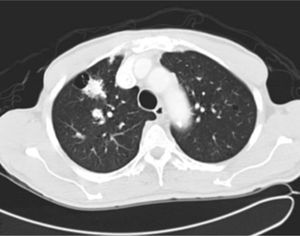
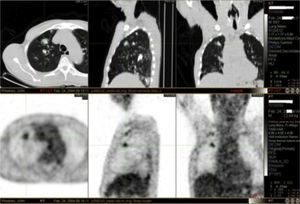
It was decided to follow up the patient with close monitoring and repeat radiological imaging. He was recalled again in two months. Since progression of the nodules was suspected (Figs. 3 and 4), a PET scan was performed on this occasion to see any uptake (see Fig. 5).
Follow-up CT scan same level as Fig. 2.
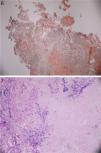
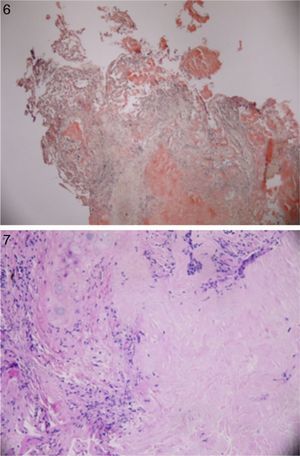
Patient underwent an unremarkable bronchoscopy and a following open wedge resection of the parenchymal lung lesion. Pathology revealed deposit of pink material, double arrows on either side of bronchial cartilage (arrow) under low magnification (see Fig. 6) and further deposits of amyloid in bronchial and alveolar tissue including vessel walls with areas of dystrophic ossification. No malignant cells were seen (Fig. 7 with congo red stain).
DiscussionWhile the presence of nodular amyloid deposits in systemic AL disease is controversial, the presence of nodular amyloid is well established. Three forms of pulmonary disease exist in localized AL amyloid: (1) nodular opacities, (2) diffuse opacities, and (3) tracheobronchial disease. A 1983 literature review of localized amyloid lung disease identified 126 cases only, 44% having nodular disease, 3% with diffuse opacities, and 53% exhibiting tracheobronchial amyloid.8,9
Among these the presence of amyloid nodules opacification on chest imaging or histologic sampling almost uniformly indicates localized disease. The incidence of nodular disease is unclear because many are diagnosed incidentally at open lung biopsy or at autopsy. Among 223 autopsies of patients with amyloidosis between 1889 and 1979, only three cases with isolated nodular lung disease were identified.10 Utz et al.11 reported seven cases of nodular disease over a 13-year period, whereas Hui et al.12 described 25 patients with lung nodules in the absence of plasma cell dyscrasia. The lesions range from 0.6 to 9cm (mean of 3cm) in size, present as multiple nodules of varying size in 58% of cases, and often appear peripherally in the lower lobes.12,13 The amyloid deposit is typically surrounded by plasma cells, lymphocytes, and giant cells; small amounts of amyloid can be found in contiguous blood vessels.10,12
Dystrophic calcification and bone formation were seen in over one third of lesions.6 Amyloid nodules grow slowly, if at all, and can cavitate.13–15 Unless densely calcified, all nodules should expand at similar rates. Dyssynchronous growth of one lesion warrants investigation to rule out carcinoma. Typically, nodular disease does not impair lung function nor impact survivorship.12
The pathogenesis of organ involvement in AL disease, be it systemic or localized, arises from the deposition of monoclonal κ or λ immunoglobulin (Ig) light chains. In systemic disease, clonally expanded plasma cells residing in the bone marrow secrete excessive quantities of monoclonal Ig, which circulates in blood to target organs. Typically AL deposits are composed of the variable region of λVI or κI light-chains. Less commonly, part or the entire Ig constant region is represented. Genotyping of these two light-chain subgroups suggests that genetic rearrangements may underlie production of amyloidogenic proteins in AL disease. Amyloid deposition is widespread in AL disease, although only specific organs may be clinically involved.16
Amino acid analyses of localized amyloid deposits reveal AL protein with κI and λIII light-chain subgroups predominating.17–19 In contrast to systemic disease, monoclonal proteins constituting localized amyloid deposits arise from a small number of plasma cells surrounding the lesion. Skin, urethra and urinary bladder, eye, larynx and supraglottic area, tracheobronchial tree, and lung parenchyma are sites most commonly involved by localized amyloid. Monoclonal light-chain does not circulate or deposit outside the target organ.
Radiologically among 28 cases of amyloid nodules, 29% calcified and 11% cavitated.20,21 Pickford et al.22 reported CT figures of five patients with localized nodular AL; 60% had one nodule, 20% had two nodules, and 20% had 10 nodules. A separate series observed multiple nodules in 67% of the cases.12 Eighty percent of the nodules had smooth contours and were located in the subpleural region of the midzone; 20% of the nodules were spiculated. No associated adenopathy or pleural disease was detected. With diffuse variety of AL disease reticular and reticulonodular patterns are reported,12 although peripheral alveolar opacities occur as well. Despite reports of reticulonodular opacities in localized AL, this X-ray pattern should prompt thorough investigations for primary systemic (AL) disease. With tracheobronchial variety of AL disease chest X-rays are normal in 50% of cases. Findings in other cases include atelectasis or lobar collapse, calcified extraluminal amyloid deposits, bronchiectasis, or hilar adenopathy.23 CT, unlike bronchoscopy, demonstrates the full extent of disease: (a) airway wall thickening, (b) irregular narrowing of airway lumen, and (c) heterotopic calcification of amyloid deposits in airway walls.22–25 Additionally, CT depicts airways beyond critical narrowing, affording full assessment of the airway.
PET imaging is a useful diagnostic and staging tool in the evaluation of suspected or known carcinoma. FDG (18 F- fluorodeoxyglucose) enters tumor cells as a consequence of increased cellular and metabolic activity. As with other diagnostic modalities, false positives can occur in cases of tuberculosis, aspergillosis, histoplasmosis and inflammatory disorders. Even though all agree the rate of false positive is low one should be aware of other possible clinical entities that can complicate the specificity of PET scanning.26 We acknowledge that FDG as a radiotracer may be insensitive to detecting amyloid which would explain the above rare finding. Furthermore, one could speculate that radiotracers used specifically to detect amyloid e.g. C11-PIB, may have clinched the diagnosis but their current use is restricted to research settings only.
The published data addressing prognosis and treatment of amyloid localized to the lung are anecdotal. Nodular disease may slowly progress, with increasing size or number of lesions, but does not impact on lung gas exchange or patient survival. Diffuse parenchymal involvement can impair lung physiology and lead to death. Rubinow et al.13 reported on four patients with diffuse lung involvement, three of whom died of respiratory failure. Corticosteroids had no effect on the course of disease in two of these patients. Systemic chemotherapy is not advocated for treatment of localized disease; however, diffuse parenchymal involvement may warrant consideration of aggressive therapy given its inexorable course.
Supportive care should be directed at minimizing mucous production, which may further compromise airway lumen caliber. Antibiotics, regular nebulizer use, and occasional courses of oral or inhaled glucocorticoids are useful adjuncts to airway debridement.