Diagnosis of tuberculous pleurisy (TP) may be challenging and it often requires pleural biopsy. A tool able to increase pre-test probability of TP may be helpful to guide diagnostic work-up and enlargement of internal mammary lymph node (IMLN) has been suggested to play a potential role.
The aim of the present investigation was to assess role of IMLN involvement in TP in a multi-centric case-control study, by comparing its prevalence and test performance to those observed in patients with infectious, non-tuberculous pleurisy (NTIP), and in controls free from respiratory diseases (CP).
MethodsA total of 419 patients, from 14 Pulmonology Units across Italy were enrolled (127 patients affected by TP, 163 affected by NTIP and 129 CP). Prevalence, accuracy and predictive values of ipsilateral IMLN involvement between cases and control groups were assessed, as well as concordance between chest computed tomography (CT scan) and thoracic ultrasound (TUS) measurements.
ResultsThe prevalence of ipsilateral IMLN involvement in TP was significantly higher than that observed in NTIP and CP groups (respectively 77.2%, 39.3% and 14.7%). Results on test performance, stratified by age, revealed a high positive predictive value in patients aged ≤50 years, while a high negative predictive value in patients aged >50 years. The comparison between CT scan and ultrasound showed moderate agreement (Kappa=0.502).
ConclusionsEvaluation of IMLN involvement plays a relevant role in assessing the pre-test probability of TP. Considering the increasing global prevalence of mycobacterial infections, a tool able to guide diagnostic work-up of suspected TP is crucial, especially where local sources are limited.
Tuberculous pleurisy (TP) is the second most common form of extrapulmonary tuberculosis.1 It manifests with a unilateral, exudative effusion and it usually presents as acute illness, especially in young immunocompetent patients; dyspnoea, fever, cough and pleuritic chest pain are the most common symptoms.2 Other clinical features include night sweats, weight loss and malaise.3
The pathogenesis of TP is linked to a delayed hypersensitivity response to mycobacterial antigens in the pleural space and, less frequently, to a direct pleural infection. Mycobacterial antigens interact with CD4+ T-lymphocytes, leading to a delayed hypersensitivity reaction, with a consequent cytokine cascade and macrophage activation, causing increased capillary permeability and diminished lymphatic drainage, and, thus, pleural fluid accumulation.4 The fact that the pathogenesis is due to the activation of the immune system is clearly visible in the immune reconstitution inflammatory syndrome, an insidious and potentially serious complication of opportunistic infections in immunocompromised patients when an effective immune response recovers.5
Diagnostic work-up of TP is usually challenging, as sensitivity from microbiological tests of pleural fluid, including both direct microscopic examination and cultures, ranges between 10 and 30%,6 although biomarkers, such as pleural adenosine deaminase (ADA), and polymerase chain reaction (PCR), might be helpful in this context.7,8 Other complementary diagnostic tests include the Mantoux test, which is often unavailable and unreliable,9-13 or interferon gamma release assays (such as QuantiFERON-TB Gold), which provide false negative results in almost 30% of the patients,14 albeit Fukushima et al. recently reported preliminary results about performance of the QIAreachTM QuantiFERON-TB assay on 41 active pulmonary tuberculosis patients, showing promising data.15 As a result, pleural biopsy, obtained via medical thoracoscopy or transthoracic approach, with direct isolation of Mycobacterium tuberculosis and demonstration of caseating granulomas, remains the gold standard. However, it is a relatively invasive technique, with a not negligible rate of false negative cases reported in literature (15-20%).16
In this context, a tool able to impact the pre-test probability of TP may be helpful to guide diagnostic work-up of these patients. With this purpose, involvement of ipsilateral internal mammary lymph node (IMLN) has been investigated in a small number of studies,4,17-20 suggesting a potential role of such clinical feature.21 Due to their peculiar anatomical location,22,23 IMLN may serve as “sentinel” of pathological processes affecting the pleura, as they are easy to detect with both thoracic ultrasounds (TUS)4 and chest computed tomography (CT scan), if enlarged;24 for this reason they are currently used in the staging of breast cancer, since their presence or absence changes the N category,25,26 and mesothelioma.27 However, evidence supporting IMLN involvement as a specific feature of TP only are currently lacking, as it has been occasionally described also in other, non-tubercular, infectious pleurisy (NTIP).
Therefore, the aim of the present investigation is to assess role of IMLN involvement at CT scan in diagnostic work-up of TP in a large multi-centric case-control study, by comparing its prevalence across different subgroups, i.e. patients affected by TP (TP group), by NTIP (NTIP group) and by other non-respiratory conditions (named control patients, CP group).
MethodsFourteen Pulmonology Units across Italy were involved in this large multi-centre observational case-control study. The study protocol complies to the ethical guidelines of the 1975 Declaration of Helsinki, and it was notified and approved by the coordinator ethics committee (approval number NP4406) and by each local ethics committee. Every center included in this study collected permission forms to the use of anonymized personal data and a specific form was also provided to patients included in this study, when possible. A waiver of informed consent form was applied in case of patient death, patients already discharged and lost at follow-up, according to our Ethical Committee indications.
Cases were consecutive patients, aged ≥18 years, with confirmed diagnosis of TP between January 2010 and December 2020. A confirmed diagnosis was defined as a case with at least one of the following diagnostic features: i) positive cultural tests; ii) Mycobacterium Tuberculosis identification at PCR or direct microscopic examination; iii) compatible histological pattern.
Cases were compared to two different control groups. The first group included patients affected by NTIP (NTIP group) with symptoms of acute inflammation (e.g. fever, fatigue, chest pain, elevated C-reactive protein).
The second group included subjects who were admitted to emergency departments for traumas or other non-respiratory medical reasons, without a history of malignancy and acute or chronic respiratory disorders (CP group), and who had at least one CT scan performed in their diagnostic work-up.
An internal mammary lymph node was considered as pathological when the short diameter was ≥5 mm, according to previous experiences.4,21 All CT-scans were evaluated by each centre's investigators along with their radiology department. All CT scans performed in TP and NTIP groups included the intravenous contrast (except for a tiny minority of patients who had contraindications, such as renal failure or previous allergy to contrast), whereas in CP group the choice of using contrast was based on indications (i.e. the underlying conditions) and local protocols. For all CT scans, slice thickness was ≤1.5 mm.
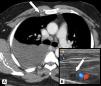
Selected demographic factors and clinical characteristics were collected for all cases and controls. These included: age, gender, laterality of pleural effusion, presence or absence of ipsilateral and/or contralateral internal mammary lymph nodes and/or mediastinal lymph nodes with relative sizes; if TUS was available, each centre was also asked to provide a TUS assessment of IMLN, in order to compare TUS and CT scan findings; concordance was defined as the concordance on number of patients with IMLNs at both TUS and CT scan (defined as detection of a lymph node with short axis ≥5 mm) (Fig. 1). Results were then provided to the coordinating centre for statistical analysis.
The primary outcome was the comparison of prevalence of ipsilateral IMLN involvement defined as at least one lymph node with the short axis ≥5 mm at CT scan, between TP group and the two control groups (NTIP group and CP group). Secondary outcomes included assessment of sensitivity, specificity, accuracy, positive and negative predictive values of IMLN involvement and concordance between CT and TUS measurements.
Statistical analysis was performed using SPSS® software (IBM, Armonk, NY, U.S.A.) and GraphPad Prism® (GraphPad Software Inc., San Diego, CA. U.S.A.). Continuous variables were compared using Kruskal-Wallis test, whereas categorical variables were compared by using chi-squared test and Fisher's exact test. Pearson's chi-squared test was used to assess the frequency distribution of the ipsilateral and contralateral IMLNs, as well as the mediastinal lymph nodes among the three groups of patients. Receiver operating characteristic (ROC) curves were built to identify potential lymph node size cut-offs. The quantification of agreement between CT scan and US was evaluated by calculating Cohen's kappa coefficient. For all tests, a p-value ≤0.05 was considered statistically significant.
ResultsA total of 419 patients from 14 centres were enrolled in the present study: 127 patients affected by TP, 163 affected by NTIP and 129 CP.
The main characteristics of study population, including the prevalence of IMLN ipsilateral to pleural effusion at CT scan in cases and in control groups and relative mean sizes are reported in Table 1. Mean age of the TP group was significantly lower as compared to the two control groups (p-value <0.001), whereas there was no significant difference in terms of sex or laterality of pleural effusion. TUS evaluation was available in 31.5% (40/127) of TP group, 22.1% (36/163) of NTIP group and in 3.9% (5/129) of CP group.
Main patients’ characteristics.
Continuous variables are reported as mean ± standard deviation. IMLN: internal mammary lymph node. TP: tuberculous pleurisy. NTIP: non-tubercular infectious pleurisy. CP: control patients. TUS: thoracic ultrasound.
With reference to NTIP group, bacterial isolation was possible in one third of patients (among them, Streptococcus genus accounted for about 33% of infections). Patients from CP group were admitted to emergency department mainly for trauma.
Overall, the prevalence of ipsilateral IMLN was 77.2% in TP group (98/127), 39.3% in NTIP group (64/163), and 14.7% CP group (19/129) (p-value <0.001). The mean sizes of ipsilateral IMLN were also significantly different between TP group, NTIP and CP group, being respectively 9.8 ± 3.8, 5.3 ± 3.3, and 2.8 ± 2.1 mm (p-value <0.001). Contralateral IMLN were present respectively in 10 (7.9%) subjects in TP group, in 4 (2.5%) subjects in NTIP group and 2 (1.6%) subjects in CP group (p-value = 0.015), whereas enlarged mediastinal lymph nodes were detected in 80 (63.0%) subjects in TP group, in 114 (69.9%) subjects in NTIP group and in 14 (10.9%) in CP group (p-value <0.001). Collateral findings in the CT scans from TP group included tree-in-bud pattern and consolidations (about one third of CT scans).
Sensitivity, specificity, accuracy, and positive and negative predictive values of ipsilateral IMLN involvement were respectively 76.4%, 78.5%, 77.6%, 73.5% and 81.0% when referring to NTIP group, whereas they were 76.4%, 98.4%, 87.5%, 98.0% and 80.9% when referring to CP group (Table 2).
Ipsilateral internal mammary lymph nodes (IMLN) in cases versus control groups.
| TP vs NTIP | |||
|---|---|---|---|
| ≤50 years | >50 years | All | |
| Sensitivity | 75.6 | 75.0 | 76.4 |
| Specificity | 75.0 | 79.5 | 78.5 |
| Accuracy | 75.4 | 78.4 | 77.6 |
| Positive predictive value | 87.8 | 53.6 | 73.5 |
| Negative predictive value | 56.3 | 91.0 | 81.0 |
| TP vs CP | |||
|---|---|---|---|
| ≤50 years | >50 years | All | |
| Sensitivity | 75.6 | 75.0 | 76.4 |
| Specificity | 98.3 | 98.6 | 98.4 |
| Accuracy | 84.7 | 90.1 | 87.5 |
| Positive predictive value | 98.5 | 87.5 | 98.0 |
| Negative predictive value | 73.1 | 90.1 | 80.9 |
All numbers are reported in percentages. IMLN: internal mammary lymph node. TP: tuberculous pleurisy. NTIP: non-tubercular infectious pleurisy. CP: control patients.
Considering the significant difference in mean age between groups (particularly the upper quartile age value of TP group, which was 53.7 years) and the mean age (<50 years) of TP reported in literature,28 we decided to stratify the analysis according to age, using 50 years of age as cut-off (under or equal 50 vs. over 50). Positive predictive value increased in the ≤50 years group, whereas negative predictive value decreased when referring to both control groups; conversely, in the >50 years group, the negative predictive value increased and the positive predictive value significantly decreased (Table 2).
To explore the role of dimensional cut-off value, we built two ROC curves (Fig. 2) in order to identify a possible dimensional cut-off value: the comparison of TP versus NTIP group resulted in a cut-off value of 12.1 mm (sensitivity 20.4%, specificity 98.4%, AUC 0.818, likelihood ratio 13.1, p-value <0.001), whereas the comparison of TP group and CP group resulted in a cut-off value of 4.6 mm (sensitivity 99.0%, specificity 88.2%, AUC 0.955, likelihood ratio 8.4, p-value <0.001).
A-B. ROC analysis for IMLN dimension with a cut-off value of 12.1 mm in comparison between TP group and NTIP group (A) and with a cut-off value of 4.6 mm in comparison between TP group and CP group (B). ROC: receiver operating characteristic. IMLN: internal mammary lymph node. TP: tuberculous pleurisy. NTIP: non-tubercular infectious pleurisy. CP: control patients.
Lastly, we compared the results obtained via CT scan to those obtained with TUS, observing a moderate agreement between them (Kappa=0.502, 95% CI 0.314-0.689, p-value <0.001).
DiscussionThe present study is the first large multi-centric case-control study, to date, assessing prevalence of ipsilateral IMLN involvement in patients with TP as compared to those with NTIP and to controls free from respiratory diseases. Overall, we found that prevalence of ipsilateral IMLN (defined as detection of a lymph node with short axis ≥5 mm at CT scan) in TP patients was significantly higher than that observed in both control groups. Although not pathognomonic, the presence of a pathological ipsilateral IMLN substantially increases the pre-test probability of TP, especially in younger subjects (aged equal to or less than 50 years), while its absence nearly permits exclusion of any infectious pleurisy (tuberculous and non-tuberculous) in all subjects, and significantly reduces the pre-test probability of TP in patients older than 50 years. The relevant impact of age on test performance is likely due to differences in prevalence of TP and NTIP in young and middle-aged subjects as compared to the elderly. According to epidemiological studies, TP is more common in younger populations,28 usually immigrants, while NTIP occurs mainly in the elderly,29 as established independent risk factors for non-tubercular empyema include older age and selected pre-existing comorbidities, more frequent in ageing population.24 In our study, indeed, a 20-year difference in mean age between TP and NTIP groups has been observed (41.9 vs 61.4).
Moreover, our findings contributed to confirm the validity of dimensional cut-off of 5 mm for distinguishing between pathological and physiological IMLN, as documented by mean of sizes observed in TP, NTIP and CP groups (respectively 9.8, 5.3 and 2.8 mm) and by the two ROC curves. To date, no consensus exists on proper dimensional cut-off for defining pathological IMLNs, although 5 mm was already employed in previous investigations.30 Our study firstly provides data on size of IMLN in a large cohort of controls free from pleurisy and other respiratory conditions, that was lower than 5 mm (2.8 ± 2.1), confirming that the cut-off for pathological lymph node should be different according to anatomical district.31 In addition, our results on mean size of IMLN in cases and controls also showed that lymph nodes in TP group were overall larger than those of NTIP group, often exceeding 10 mm. Therefore, the presence of an IMLN with a short axis greater than 10 mm should further raise suspicion of an underlying tubercular etiology.
Finally, another important finding from our study is the significant, although not excellent, concordance between CT and TUS evaluations. The choice of assessing IMLN prevalence by means of CT scan was due to the higher diffusion and the lower variability in measurements of this technique as compared to TUS, that is more likely to be influenced by operator experience and it is not available in every centre. However, TUS offers the advantage of being non-ionising, quicker, easily available at patient's bedside and in outpatient setting, and therefore, in presence of adequate skills, may be the first approach to assess IMLN involvement, reserving a more accurate CT scan evaluation with relative measurements to patients with suspected pathological lymph nodes.
Involvement of IMLN has been already investigated in a few previous studies by means of CT or TUS, overall reporting a significant association, but none has compared its prevalence to control groups, and its predictive value in diagnostic work-up of TP.
As previously underlined, diagnosis of TP is a challenge for physicians, as clinical and radiological features are often aspecific, microbiological analyses on pleural fluid are affected by a significant false negative rate, and tissue acquisition, essential for Mycobacterium tuberculosis identification, is frequently delayed, due to the higher risks and the limited availability of technical equipment and skills, with relevant implications to patient prognosis. In this context, presence of IMLN involvement, although not diagnostic per se, may be extremely helpful in guiding subsequent diagnostic steps in patients with suspected TP, as it significantly increases the pre-test probability of the disease, especially in younger subjects, suggesting the need of a prompt bioptic approach. On the contrary, in older patients, the absence of IMLN involvement might allow clinicians to avoid invasive procedures with less favourable risk-benefit profile.
Major strengths of the present study include the large number of cases, the comparison with two different control groups and the representativeness of study population, as different centres across Italy were involved. On the other hand, a number of limitations must be acknowledged. First, cases and controls were not matched for age and sex by study design, due to difficulties in recruitment across the centres included. However, subsequent analyses according to age subgroups allowed us to minimize this methodological limit. Moreover, TUS evaluations were not performed in every centre (<20% of subjects), and different operator experience, when carried out, might have influenced data on concordance with CT scan.
ConclusionsIn conclusion, the present study firstly provides evidence that assessment of IMLN involvement (via CT scan and possibly TUS) is a key element to guide diagnostic work-up of patients with suspected tubercular pleural effusion, although not pathognomonic. In the case of presence of a pathological IMLN, the pre-test probability of mycobacterial origin is substantially increased, especially in young subjects and when lymph nodes are larger than 10 mm, strengthening the indication for more invasive diagnostic approaches. On the contrary, its absence (defined as a IMLN short axis smaller than 5 mm), particularly in patients over 50 years of age nearly excludes an underlying TP.
Considering the increasing global prevalence of tuberculosis, a tool able to guide diagnostic steps in this challenging scenario is of utmost importance, especially in subjects with a less favourable risk-benefit profile of a more invasive approach and where local sources are limited.
Author contributionsGL and GM: study design. All authors: data collection. GL: data analysis. GL, GM, CR and FM: data interpretation. GL, CR, and FM: wrote the first manuscript draft. All authors: reviewed and approved the final version of the manuscript.
Financial/nonfinancial disclosuresThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Other contributionsThe authors wish to thank all “Pleural-Hub” group affiliates for their continuous support, interest and enthusiasm. Moreover, the authors wish to thank Dr. Cesare Tomasi for data analysis and Mrs Mary Elizabeth Orme for English language editing.
Data availability statementThe data that support the findings of this study are available from the corresponding author upon reasonable request.