Purpose of the research: transbronchial lung cryobiopsy has been recently accepted as a valid and less invasive alternative to surgical lung biopsy. The purpose of this randomized controlled study was to evaluate, for the first time, the quality and safety of biopsy specimens obtained by using the new disposable 1.7-mm cryoprobe compared with the standard re-usable 1.9 mm cryoprobe in the diagnosis of diffuse parenchymal lung diseases. Methods: 60 consecutive patients were prospectively enrolled and randomly assigned to two different groups: 1.9 mm (group A) and 1.7 mm (group B); primary endpoints were pathological and multidisciplinary diagnostic yield, sample size and complication rate. Principal results: the pathological diagnostic yield of cryobiopsy was 100% in group A and 93.3% in group B (p = 0.718); cryobiopsy median diameter was 6.8 mm in group A and 6.7 mm in group B (p = 0,5241). Pneumothorax occurred in 9 patients in group A and 10 in group B (p = 0.951); mild-to-moderate bleeding in 7 cases and 9 cases in group A and B respectively (p = 0.559). No death or severe adverse events were observed. Conclusions: there was no statistically significant difference between the two groups, regarding diagnostic yield, adverse events and sampling adequacy.
Transbronchial lung cryobiopsy (TBLC) has been recently accepted as a valid and less invasive alternative to surgical lung biopsy (SLB) in diagnosis of interstitial lung diseases (ILDs).1 More recently, European Respiratory Society (ERS) guidelines have been published providing evidence-based clinical practice recommendations on the role of TBLC in patients with undiagnosed ILDs.2 Transbronchial lung cryobiopsy allows obtaining of larger and higher quality lung tissue samples without the crush artefacts seen with conventional transbronchial lung biopsy using flexible forceps. New single-use cryoprobes have been recently developed with outer diameters of 1.1 mm, 1.7 mm and 2.4 mm to replace the previous re-usable 1.9 mm and 2.4 mm probes. Diagnostic yield, complications rate and sample artefacts related to these different cryoprobes used in interstitial lung diseases have not been assessed yet.3 The purpose of this randomized controlled study was to evaluate the quality and safety of biopsy specimens obtained using the new disposable 1.7 mm cryoprobe compared with the standard re-usable 1.9 mm cryoprobe used in the diagnosis of diffuse parenchymal lung diseases.
MethodsThis prospective, randomized study was performed in Italy at the Pulmonology Unit of GB Morgagni Hospital. 60 consecutive patients with suspected diffuse parenchymal lung disease on high resolution computed tomography (HRCT) requiring histological examination for further evaluation were prospectively enrolled between 1st January 2021 and 30th June 2021. The following clinical data were extracted: age, gender, past medical history, medications, smoking history, environmental exposure history, physical examination findings, laboratory results (including antinuclear autoantibodies, autoantibodies against ex-tractable nuclear antigens, anti-neutrophil autoantibodies, and precipitins), and pulmonary function (forced vital capacity - FVC% predicted and carbon monoxide diffusion capacity – DLCO% predicted). HRCT images were made within one month before bronchoscopy and CT scans were analyzed by one dedicated radiologist (S.P.). The study was approved by CEROM (Comitato Etico della Romagna, prot. 7992/2020). Written informed consent for participation in the study was obtained from each patient. All patients were over 18 years old. Exclusion criteria included coagulopathy (thrombocytopenia < 70.000/μl, prothrombin time international normalized ratio >1.5), FVC 〈50% of the predicted value, DLCO < 30% of the predicted value and FEV1 <0.8 L, diffuse bullous disease, hemodynamic instability, echocardiographic pulmonary arterial systolic pressure 〉 50 mmHg and severe hypoxemia (PaO2 < 55 mmHg on room air).
A 1.9 mm or 1.7 mm cryoprobe was used (Erbokryo CA; Erbe Elektromedizin GmbH, Tübingen, Germany). The enrolled patients underwent transbronchial lung cryobiopsy (TLCB) and were randomly assigned to two different groups: group A (1.9 mm probe was used) and group B (1.7 mm probe). The study aimed to evaluate safety and efficacy of new disposable 1.7 mm cryoprobe compared with the standard re-usable 1.9 mm cryoprobe. Primary endpoints were: 1) pathological diagnostic yield, 2) final multidisciplinary diagnosis, 3) sample size (maximum and minimum axis of the specimen at the microscope), and 4) complication rate (pneumothorax, bleeding). Secondary endpoints were: 1) histological artifacts, and 2) incapacity to retrieve material. For each biopsy, if no samples were obtained after three attempts, the biopsy was rated as failed.4 Artifacts included bronchial clefts in the alveoli and bleeding. The switch from one probe to another by the operator was allowed in case of difficulty in sampling; indeed, this was one of the data collected and analyzed.
Two experienced operators (V.P and C.R.) performed bronchoscopies as previously described. Patients were deeply sedated (using propofol and remifentanil), maintained in spontaneous breathing and intubated with a rigid tracheoscope (Karl Storz GmbH, Tuttlingen, Germany). Biopsies were obtained under fluoroscopic guidance at a distance of approximately 10 mm from the thoracic wall; sampling was targeted to the areas of abnormality seen on HRCT, with samples taken from one site or multiple sites depending on the radiological pattern and distribution of disease, as for clinical practice; in particular, cryobiopsy was performed in different sites in patients with significant radiographic inter-lobar heterogeneity, while in patients with diffuse radiographic pattern (both in the upper and the lower lobes) or in patients with a significant apical, basal gradient, cryobiopsy was more frequently performed in the same lobe. The choice of the site and side of biopsy was decided before the procedure. The probe was cooled for approximately 7–8 s or 9–10 s for the 1.9 mm and 1.7 mm diameter respectively. A Fogarty balloon was always routinely used to prevent severe bleeding. As previously described,5 bleeding was defined as “mild” if requiring just endoscopic aspiration, “moderate” if requiring further endoscopic procedures (bronchial occlusion and/or instillation of ice-cold saline), and “severe” if requiring surgical interventions, transfusions and/or admission to intensive care unit for hemodynamic or respiratory instability. Within three hours of the procedure, a chest radiograph was performed to assess for pneumothorax.6
Specimens were reviewed by two dedicated lung pathologists (A.D. and V.P.). Biopsies were considered “non-diagnostic” when histopathologic criteria sufficient to define a characteristic histopathologic pattern were lacking (e.g., normal lung or minimal nonspecific changes) or when samples were considered inadequate (e.g., too small or airway wall with no alveolated lung parenchyma). Clinical information, radiological features and biopsy results were then reviewed by clinicians, radiologists and pathologists. A multidisciplinary diagnosis was made, with cryobiopsy considered diagnostic if additional evaluation, including surgical lung biopsy, was considered unnecessary.
Statistical analysisStatistical analyses were carried out using SPSS Statistics. Data are reported as median and range for continuous variables and as number and percentage for discrete variables. The different approaches to sampling (groups A and B) were compared using standard statistical approaches (the McNemar χ 2 or Fisher exact text for categorical variables and the Mann-Whitney U test for continuous variables). A p value of <0.05 was defined as representing a statistically significant difference.
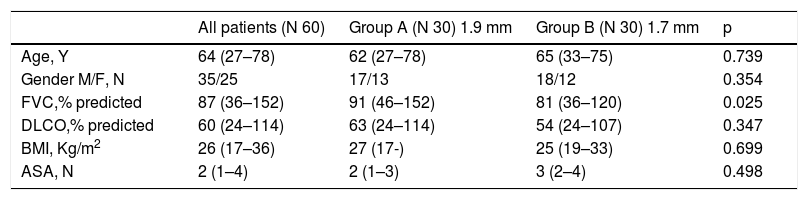
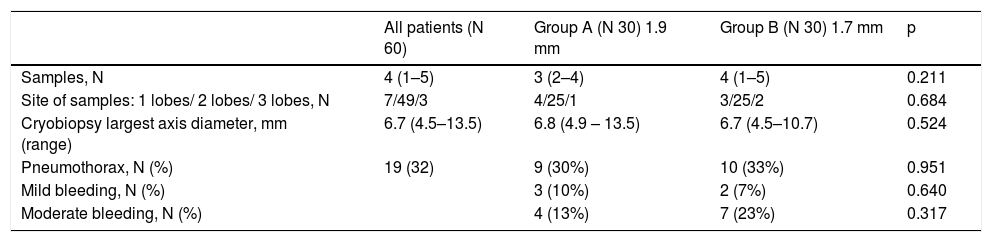
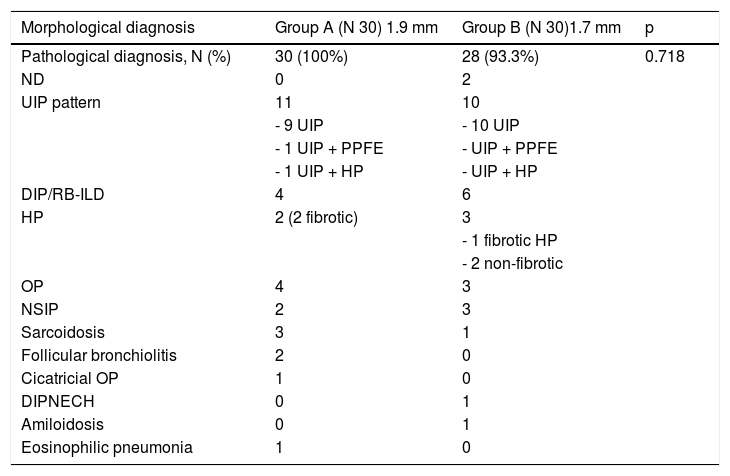
Results60 subjects with suspected ILD underwent cryobiopsy for evaluation of diffuse parenchymal lung disease. Median age was 64 years (range 27–78), 35 male and 25 female, median baseline forced vital capacity (FVC) was 87% (range 35–152), median baseline diffusing capacity of the lungs for carbon monoxide (DLCO) was 60% (range 24–114). Clinical features of the patients are reported in Table 1. 30 patients underwent transbronchial lung cryobiopsy with a 1.9 mm probe (group A) and 30 patients with a 1.7 mm probe (group B). Cryobiopsy smallest axis diameter was 3,75 mm (range 2,0–4,6 mm) in group A and 3,0 mm (range 1,0–5,0 mm) in group B, whereas cryobiopsy largest axis diameter was 6,8 mm (range 4,9 - 13,5 mm) in group A and 6,7 mm (range 4,5–10,7 mm) in group B (Table 2). There was no statistically significant difference in size regardless of whether the biopsy was performed with the 1.7 mm or with the 1.9 mm probe (p = 0,5241) (Table 2). In group A, the patients underwent three biopsies on average (range 2–4), from two different sites in the majority of cases (25 patients), from one single site in 4 cases, from three different sites in one patient; in group B, the patients underwent four biopsies on average (range 1–5), from two different sites in 25 cases, from one single site in three cases, from three different sites in two cases (p = 0,6843) (Table 2). The pathological diagnostic yield of cryobiopsy was 100% in group A (1.9 mm probe) and 93.3% in group B (1.7 mm); there was no statistically significant relationship regardless of whether biopsies were performed with 1.7 mm probe or 1.9 mm probe (p = 0.718). The different pathologic patterns observed are described in Table 3. A multidisciplinary group discussed each case, and a final multidisciplinary diagnosis was obtained in 100% of cases in group A (1.9 mm probe), of which 26/30 with high confidence level, and in 100% in group B (1.7 mm), of which 28/30 with high confidence level.
Clinical features of patients submitted to trans-bronchial lung criobiopsy (TBLC).
Abbreviations: FVC forced vital capacity, DLCO diffusing capacity of the lungs for carbon monoxide; BMI body mass index; ASA American Society of Anaesthesiologists physical status.
Biopsy characteristics, sampling strategy and complications in patients submitted to trans-bronchial lung cryobiopsy (TLCB).
Histopathologic pictures of patients undergoing transbronchial lung cryobiopsy.
Abbreviations: ND non-diagnostic; UIP usual interstitial pneumonia; PPFE pleuroparenchymal fibroelastosis; DIP desquamative interstitial pneumonia; RB-ILD respiratory bronchiolitis-interstitial lung disease; HP hypersensitivity pneumonitis; OP organizing pneumonia; NSIP non-specific interstitial pneumonia; DIPNECH diffuse idiopathic pulmonary neuroendocrine cell hyperplasia.
In terms of safety, no severe bleeding was observed, only mild-to-moderate bleeding (7/30 cases in group A and 9/30 cases in group B) (p = 0.559) (Table 2). Pneumothorax was documented in 9/30 cases in group A and 10/30 cases in group B (p = 0.951), with only 9 patients (15%) requiring chest tube positioning; fragments of pleura were found in 10 patients in group A and 12 patients in group B. No death, acute lung injury, persistent fever, prolonged air leak, pneumonia/empyema, or other adverse events were observed after TLCB. Histological artifacts were occasionally found but no difference between the two groups (p = 0.301). Regarding difficulties in retrieving the material, in one patient from group A, sampling failed and the operator decided to switch the probe from 1.9 mm to 1.7 mm; among group B patients, the 1.7 mm probe was switched to 1.9 mm in 4 cases.
DiscussionThis is the first prospective study evaluating diagnostic yield and complications in two different randomized and homogenous groups of patients with suspected diffuse parenchymal lung diseases undergoing transbronchial lung cryobiopsy with two different size probes. The observed mean pathological diagnostic yield of cryobiopsy was 100% with a 1.9 mm probe and 93.3% with a 1.7 mm probe and there was no statistically significant difference between the two groups. The UIP pattern was identified in 11 cases in group A and 10 cases in group B. For cases identified as “fibrotic hypersensitivity pneumonitis with UIP pattern,” the diagnosis was obtained analyzing samples obtained from different segments by the presence of some ancillary features (e.g., foci of intra-alveolar organizing pneumonia, loosely formed microgranulomas, lymphocytic bronchiolitis).7,8 Extensive peribronchiolar metaplasia, particularly peribronchiolar metaplasia affecting more than half the bronchioles, supported a diagnosis of fibrotic hypersensitivity pneumonitis over usual interstitial pneumonia.9
A final multidisciplinary diagnosis was obtained in 100% of patients in both groups and there was no statistically significant relationship regardless of whether biopsies were performed with 1.7 mm probe or 1.9 mm probe (p = 0.718).
Consensus statements and guidelines dealing with the standardization of TBLC are now available10-13 and recently, the European Respiratory Society (ERS) established a task force to develop guidelines aimed at providing evidence-based clinical practice recommendations on the role of TBLC in patients with undiagnosed ILD.2,14 High confidence final diagnosis can be obtained for most patients undergoing TBLC2,15; furthermore, the percentage increase in ILD and IPF diagnosis made with high level of confidence in MDD may rise significantly before and after adding TBLC information.16,17 In the Troy paper, a diagnostic pattern was obtained in 90.8% for TBLC, with a histopathological agreement (for guideline-refined pattern) with surgical lung biopsy of 70.8%, and a weighted kappa agreement of 0.70 (95%CI 0.55–0.86)15; similar results were found in other indirect comparative studies (TBLC diagnostic yield 82.8%).6
We know from the literature that diagnostic yield may be significantly influenced by sampling strategy, improving dramatically when ≥2 samples are performed (instead of only one) and when biopsy is obtained in two different sites (instead of only one), either from the same lobe or from different lobes.5,6 In fibrotic lung diseases, in which pathological variability is more challenging and differential diagnosis could be more complex18; discordant samples between different sites have been described in 30% of cases.5 Recent guidelines made some recommendation about an optimal strategy to sample lung tissue2,12 and multiple biopsies are usually taken to reduce sampling error; average number of biopsies per patient in our study was 4 and sampling was always performed in different lobes in case of evident radiographic inter-lobar heterogeneity or in different segments of the same one lobe when diffuse radiographic pattern was observed both in the upper and the lower lobes or in patients with a significant apical-basal gradient.
The ideal specimen size for pattern recognition has not yet been determined in terms of size, but some pathologists recommended that appropriate specimens measure 5 mm in diameter (equal to the size of the whole field visible via a 4x objective on common microscopes).19 Different freezing durations could be required to attain the same specimen size; in our series, we applied 7 s with the 1.9 mm probe and 8 s with the 1.7 mm probe. The mean larger diameter was not related to the size of the probe (p = 0.5241), and there was no significant difference between the two groups in terms of sample size or any difference in terms of diagnostic yield between 1.9 and 1.7 mm outer diameter.
Retrospective analysis and comparison of the pathological and overall multidisciplinary diagnostic yield between two patient groups who underwent cryobiopsy with the reusable 1.9 mm probe and the reusable 2.4 mm probe in the past revealed no appreciable differences.5
It has been well demonstrated that severe complications are lower in TBLC than SLB; overall mortality rate is 0.3%.20 More importantly, TBLC appears to be safe in ILD patients in whom lung biopsy is at high-risk of complications (based on high age, BMI, lung impairment and/or cardiac comorbidities), with an equal rate of bleeding, pneumothorax, mortality and hospital stay compared to low-risk patients (based on one study with a limited number of patients21; hospitalized patients tend to have a higher risk of complications than non-hospitalized patients22,23 and mortality rates appear to be significantly higher in critically ill patients with acute hypoxemic respiratory failure, albeit it is unclear if TBLC contributed to this.24
Procedure-related factors may have an impact on complications: in particular, a higher incidence of pneumothorax had been observed when samples were taken from multiple sites, from the lower lobes and using the re-usable 2.4 mm probe as opposed to the 1.9 mm probe5; the risk of pneumothorax was also associated with the numbers of samples and the degree of lung function impairment (forced vital capacity, FVC, and diffusing capacity of the lungs for carbon monoxide, DLCO). There were 10 cases of pneumothorax in group B and 9 cases in group A in this study, and there were no differences between the two groups (p = 0.951). Contrarily, prior studies have shown that the incidence of bleeding was not correlated with the size of the probe, and our data appear to support these findings; no severe bleeding was observed, only mild-to-moderate bleeding which was not different between the groups (p = 0.559). No death, acute lung injury, or other adverse events have been observed.
In terms of artifacts, criobiopsy specimens may present artefacts (such as intra-alveolar hemorrhage or acute lung injury related to the trauma of the procedure, consisting in airspace accumulation of blood and fibrin)19; depending on the plane of sections and how the tissue is removed from the probe, the hole left in the tissue by the probe may be encountered or when the probe remains in proximity to an airway, the airway may become the major portion of the specimen or nests of airway wall may be carried into the alveolar parenchyma. Any “frozen artifact” (including slight lack of crispness of nuclear and cytoplasmic features and of connective tissue) are minor and have little effect on histologic interpretation.19 Finally, in a transbronchial cryobiopsy, one may discover parietal pleura and/or chest wall skeletal muscle. We discovered no difference between the two groups in our investigation, and histological artifacts were only found on rare occasions.
In one patient from group A, sampling failed and the operator decided to switch the probe from 1.9 mm to 1.7 mm; among group B patients, 1.7 mm probe was switched to 1.9 mm in 4 cases. Data are very limited and they do not allow for speculations, but one possible explanation could be because placement of the disposable 1.7 mm probe at the lung periphery is more difficult due to probe stiffness, particularly in cases of bronchomalacia; a prospective investigation comparing two independent randomized and homogeneous groups of patients with suspected ILD with or without bronchial malacia could corroborate these findings.
The study has some limitations, inherent to its relatively small sample size and monocentric design. A micro-cost analysis (detailed review of all costs related to a procedure) was not undertaken because it was outside the scope of this paper; the cost-effectiveness of re-usable cryoprobes versus one-use cryoprobes would also rely on cryobiopsy volume of the bronchoscopic care.
In conclusion, this is the first prospective study to compare diagnostic yield and complications in two distinct randomized and homogenous groups of patients with suspected diffuse parenchymal lung diseases undergoing transbronchial lung cryobiopsy with two different sizes probes (1.7 mm and 1.9 mm). There was no statistically significant difference between the two groups, in terms of diagnostic yield (both pathological and multidisciplinary), adverse events (bleeding and/or pneumothorax) and sampling adequacy (alveolated tissue, artifacts and biopsy size).
FundingNone of the authors received any funding for their work.
AMMP (Associazione Morgagni Malattie Polmonari).