Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) is a rare disorder, with about 200 cases described in the most recent review studies.1
We present a case of a 53-year old woman, complaining of a chronic non-productive cough and dyspnea. She had previously been assigned as a COPD patient. The patient was a non-smoker with no relevant exposure context. Pulmonary function tests demonstrated a mild obstructive pattern: FEV1 1.76L (85% of predicted); FVC 3.31L (123% of predicted); FEV1/FVC 53%. During the work-up a CT was performed showing diffuse solid pulmonary nodules (Fig. 1A), the largest in the right middle lobe (RML) measuring 18mm (Fig. 1B), and a diffuse mosaic attenuation pattern (MAP) (Fig. 1C). A comparative study was performed with a previous CT from 4 years earlier (in 2010), which revealed an increase in nodule number and size, particularly those located in the RML. Despite the suspicion of lung metastases, due to the insidious increase of nodules dimensions, a surveillance CT was suggested. The follow-up CT, performed 9 months later (in 2015) did not show any changes in the pulmonary findings. Considering the persistence and apparent non-specificity, after a multidisciplinary meeting, it was decided to biopsy the largest nodule. This coincided with the diagnosis of invasive ductal carcinoma of the left breast, which was treated with mastectomy and chemotherapy. Therefore, the transthoracic needle biopsy of the lung was postponed to 2016 and, as it shown to be a carcinoid tumour, the main concern was that a metastatic disease could not be excluded only by that biopsy. So, it was decided to perform a second biopsy, which also revealed the presence of a carcinoid tumour. Since the differential diagnosis between typical or atypical carcinoid tumours can only be performed by exeresis, it was decided to perform an atypical resection of largest pulmonary nodules. The pathologic exam disclosed features consistent with DIPNECH with tumourlets and two typical carcinoid tumours (Fig. 2). The patient recovered well from the surgery, is now under surveillance and being medicated with inhaled corticosteroids and long-acting β2-agonist for treatment of the obstructive component of the pulmonary disease.
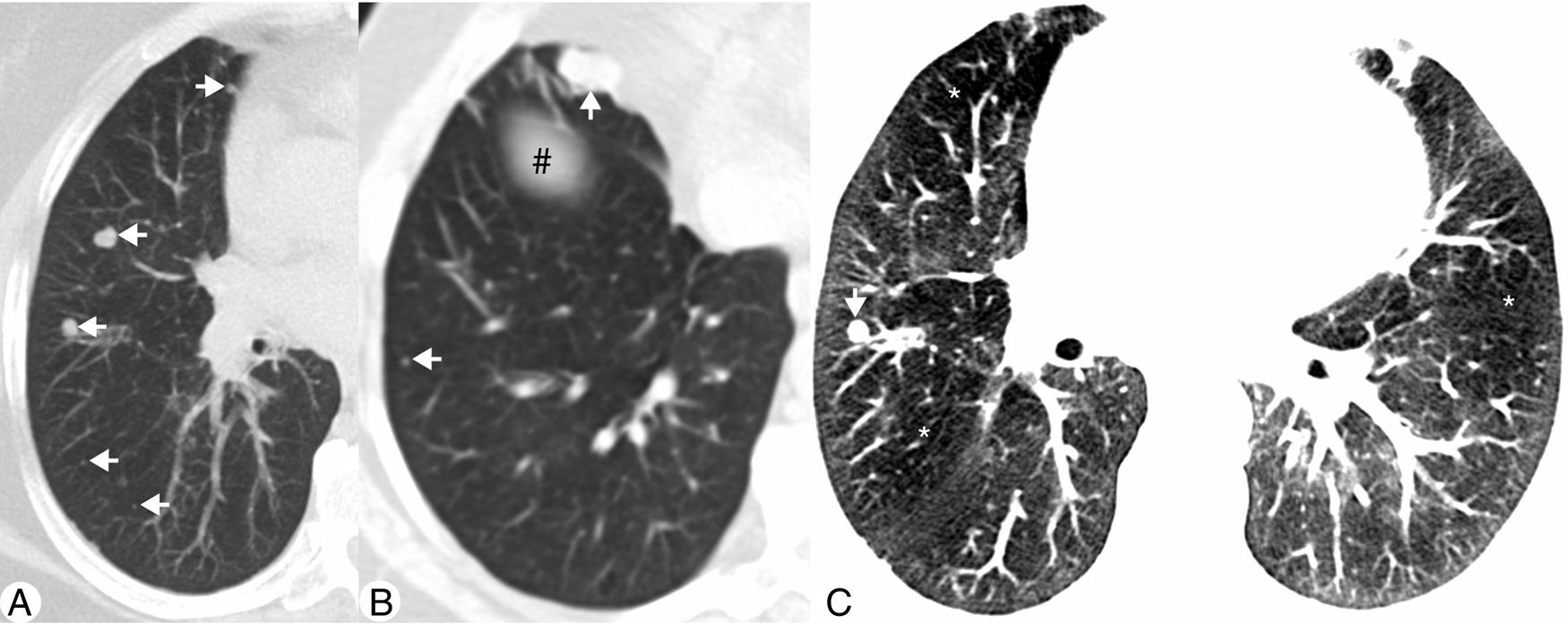
(A) Axial maximum intensity projection (MIP) CT image demonstrating multiple solid nodules located in the right middle lobe and right lower lobe (arrows). (B) Axial MIP CT image of a lower plane, which intercepts the diaphragm (#), showing other nodules (arrows), the largest in the medial segment of right middle lobe. (C) Axial CT image with narrow lung window settings demonstrating diffuse attenuation pattern with geographic areas of decreased density (*) adjacent to normal lung parenchyma. Note a small pulmonary nodule in the right middle lobe (arrow).
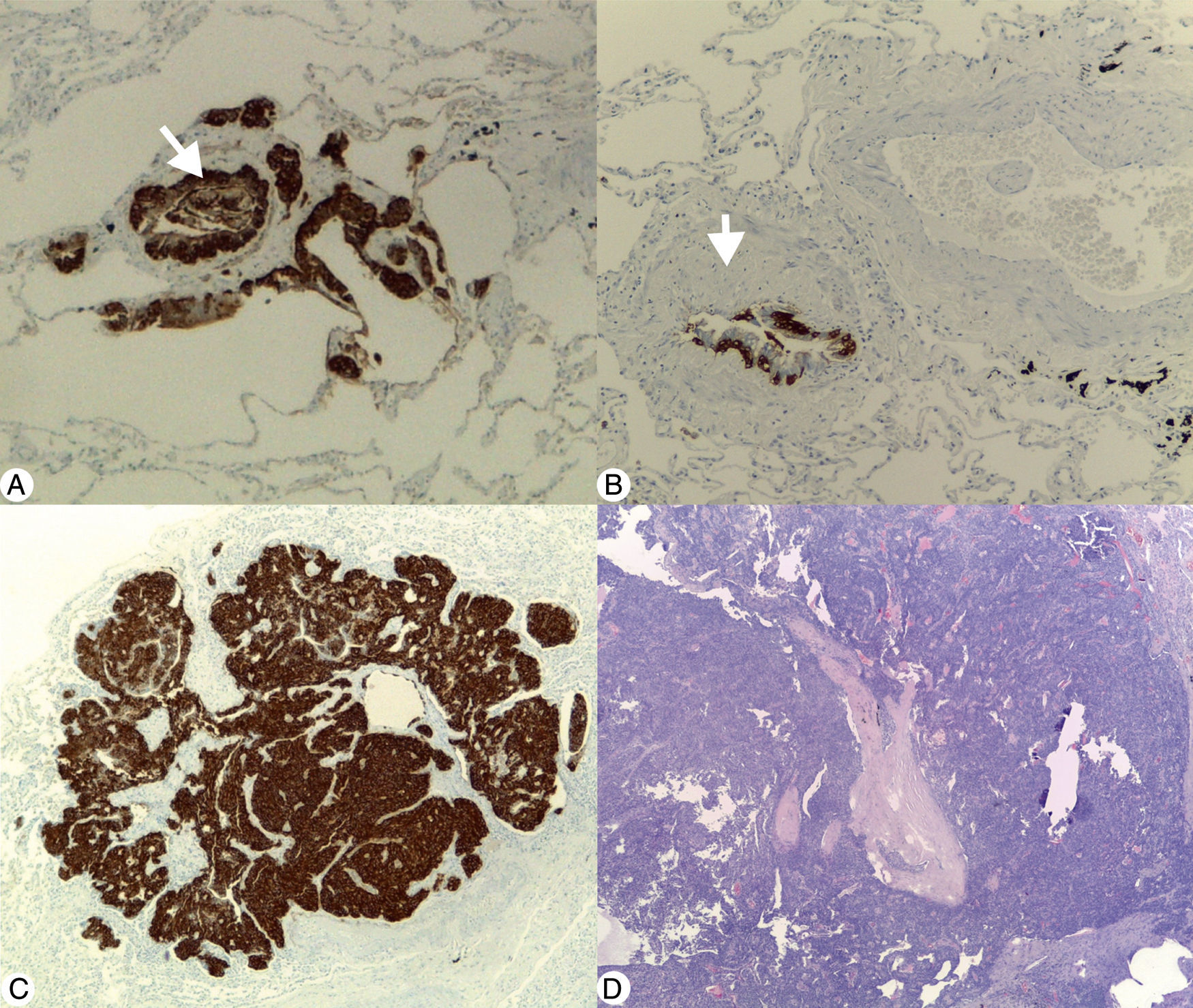
(A) Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: immunohistochemical stain (Chromogranin A 40×) demonstrating linear and nodular (arrow) neuroendocrine cell proliferation. (B) Constrictive bronchiolitis: immunohistochemical stain (Chromogranin A 100×) displaying a partial narrowing of bronchiole lumen (arrow) with linear hyperplasia of neuroendocrine cells. (C) Tumourlet: immunohistochemical stain (Chromogranin A 40×) revealing neuroendocrine cells proliferation, with spread into the peribronchiolar tissue, exhibiting a nesting pattern. (D) Typical carcinoid: low magnification photomicrograph (H&E 20×) of the medial segment of right middle lobe showing a tumour with an organoid pattern.
DIPNECH is characterized by the proliferation of pulmonary neuroendocrine cells (PNECs), usually located in bronchial wall and confined to epithelium.1,2 If these proliferations extend beyond the basement membrane, they are called tumourlets (PNECs aggregates <5mm) or carcinoid tumours (nodules >5mm).3 DIPNECH predominantly affects middle aged women. Characteristically it has an insidious onset and, by the time of the diagnosis, symptoms have often been present for several years. Persistent, chronic non-productive cough, exertional dyspnea and wheezing are the most frequently reported symptoms. Pulmonary function tests typically reveal an obstructive or mixed ventilatory defect, without a significant response to bronchodilators.1,2 Patients are usually misclassified as having asthma or COPD.
The CT abnormalities associated with DIPNECH are related to airway disease, including MAP, bronchial wall thickening, and in some cases bronchiectasis and mucoid impaction. MAP is a consequence of constrictive bronchiolitis.2 The radiological hallmark of DIPNECH is the presence of solid or ground-glass nodules, which correspond to either tumourlets or carcinoid tumours. Characteristically, the nodules demonstrate an indolent growth during the follow-up.2,3
The role of bronchoalveolar lavage is not established in DIPNECH, although some reports describe the presence of lymphocytosis.4
In the appropriate clinical (middle-aged woman with chronic cough) and radiological (pulmonary nodules with MAP) scenario, DIPNECH is the main diagnosis to be considered, however diagnosis is mainly histological and surgical biopsy is the gold standard diagnostic test.1,2
Information regarding treatment and clinical course of DIPNECH is scarce and data concerning long term follow-up are limited.1,2 It has been described that patients maintain long-term stability or a slowly progressive symptomatology and functional decline.2 Due to the indolent nature of DIPNECH, a conservative management is often adopted.2
This case reminds us that DIPNECH may mimic metastatic disease and therefore it should be considered in the differential diagnosis in patients presenting with pulmonary nodules and MAP with concordant clinical features.
FundingThe authors declare that no funding was received for this paper.
Conflict of interestsThe authors have no conflicts of interest to declare.