Chronic Mountain Sickness (CMS) syndrome, combining excessive erythrocytosis and clinical symptoms in highlanders, remains a public health concern in high-altitude areas, especially in the Andes, with limited therapeutic approaches. The objectives of this study were to assess in CMS-highlanders permanently living in La Rinconada (5100–5300 m, Peru, the highest city in the world), the early efficacy of acetazolamide (ACZ) and atorvastatin to reduce hematocrit (Hct), as well as the underlying mechanisms focusing on intravascular volumes.
Materials and methodsForty-one males (46±8 years of age) permanently living in La Rinconada for 15 [10–20] years and suffering from CMS were randomized between ACZ (250 mg once-daily; N = 13), atorvastatin (20 mg once-daily; N = 14) or placebo (N = 14) uptake in a double-blinded parallel study. Hematocrit (primary endpoint) as well as arterial blood gasses, total hemoglobin mass (Hbmass) and intravascular volumes were assessed at baseline and after a mean (±SD) treatment duration of 19±2 days.
ResultsACZ increased PaO2 by +13.4% (95% CI: 4.3 to 22.5%) and decreased Hct by -5.2% (95% CI: -8.3 to -2.2%), whereas Hct remained unchanged with placebo or atorvastatin. ACZ tended to decrease Hbmass (-2.6%, 95% CI: -5.7 to 0.5%), decreased total red blood cell volume (RBCV, -5.3%, 95% CI: -10.3 to -0.3%) and increased plasma volume (PV, +17.6%, 95% CI: 4.9 to 30.3%). Atorvastatin had no effect on intravascular volumes, while Hbmass and RBCV increased in the placebo group (+6.1%, 95% CI: 4.2 to 7.9% and +7.0%, 95%CI: 2.7 to 11.4%, respectively).
ConclusionsShort-term ACZ uptake was effective to reduce Hct in CMS-highlanders living at extreme altitude >5,000 m and was associated with both an increase in PV and a reduction in RBCV.
More than 80 million people worldwide live permanently in high-altitude areas (i.e., > 2500 m) and are exposed to chronic hypobaric hypoxia.1 Mechanisms of adaptation to this hypoxic stress include increasing levels of hemoglobin concentration [Hb], especially in native Andean populations.2,3 However, among highlanders, excessive erythrocytosis (EE, defined as [Hb] ≥ 21 g·dL−1 in men and ≥ 19 g·dL−1 in women4) can occur and lead to severe clinical symptoms characterizing the chronic mountain sickness (CMS) syndrome. CMS is highly prevalent in Andean area,2–4 and is associated with systemic vascular dysfunction5–7 and/or pulmonary hypertension8,9 and may reduce life expectancy.2,3
While several pharmacological or non-pharmacological interventions have been tested for EE and CMS, the optimal treatment for these conditions remains to be identified.10,11 Among previously tested molecules, a 250 mg once-daily dose of acetazolamide (ACZ) has been shown to be the minimal effective dose to increase ventilatory response to hypoxia and to reduce both hypoxemia and hematocrit (Hct) up to 6 months in CMS highlanders at ∼4300 m.12–15 However, the exact mechanisms by which ACZ decreased Hct remain unclear, some results in highlanders suggesting a decrease in the hypoxia-induced erythropoiesis and possibly in total hemoglobin mass (Hbmass),13,14 while an animal study suggested an expansion of plasma volume (PV), albeit non-significant compared to hypoxic controls.16 The ability of this daily low-dosage of ACZ to sufficiently increase ventilation, improve hypoxemia, and to reduce Hct in CMS patients living at higher altitudes (where partial oxygen pressure is lower) remains uncertain. The changes in Hbmass and blood volumes that may underlie this change in Hct also remain to elucidate. Statins are other molecules that could be beneficial against CMS through their pleiotropic effects on vascular function17 and possibly on erythropoiesis18 but their effects have never been properly tested in this context. Thus, we conducted in La Rinconada (a gold mining city located in southern Peru at 5100–5300 m and considered as the highest city in the world19), a double-blinded, placebo-controlled study, to assess the efficacy of ACZ and statins to decrease Hct, improve CMS condition and to understand the underlying changes in intravascular volumes.
While the final evaluations of this clinical trial (initially planned after 9 months of treatment) were disturbed by the COVID-19 pandemic,20 most of the patients included were reevaluated after 3 weeks of treatment in order to assess 1) the potential short-term efficacy of the treatments on Hct and 2) the potential underlying mechanisms of these treatments on intravascular volumes. We hypothesized that ACZ only would reduce the Hct following 3 weeks of treatment, ant that this early change would be mediated by both a decrease in Hbmass and a PV expansion.
Material and methodsStudy designWithin a large research project (Expedition5300) conducted in La Rinconada,19 CMS highlanders permanently living in the city were included in a randomized, double-blinded, parallel, placebo-controlled study after having given their written informed consent. This study was approved by the ethic committee of the Universidad San Martin de Porres, Lima, Peru (IRB00003251) and was conducted in accordance with the Declaration of Helsinki.
Participants and screeningCMS participants were recruited among the inhabitants of La Rinconada who spontaneously reported to our temporary medical research laboratory. Screening for inclusion was performed during a medical consultation conducted by a local native-Spanish speaking investigator. Male highlanders, 18 – 55 years old, native from the Altiplano area (i.e., born above 3500 m), permanently living for more than 3 years in La Rinconada, whose CMS was formally diagnosed during an initial medical visit, were invited to participate. Exclusion criteria included an history or a newly-diagnosed condition that could worsen hypoxemia and thus interfere with the CMS diagnosis4 as well as relative or absolute contra-indication to drug administration (i.e. obesity - defined as a body mass index ≥30 kg·m−2, active smoking or drug intake, any cardio-respiratory, neurological, and metabolic or kidney disease). No patients had previously been treated for CMS, neither by ACZ nor phlebotomy before inclusion. The initial diagnosis of CMS was made according to the 2005 consensus4 based on a Qinghai (or total) CMS score ≥ 6, including the presence of EE.3,4 The total CMS score is based on the evaluation of seven clinical signs or symptoms (breathlessness and/or palpitations, sleep disturbance, cyanosis, veinous dilatation, paresthesia, headache, tinnitus) from 0 (absence of symptom) to 3 (severe symptom), to which another 3 points are added in the presence of EE ([Hb] ≥ 21 g·dL−1 for men). Severity of CMS is defined as mild (total CMS score= 6–10), moderate (total CMS score= 11–14) or severe (total CMS score ≥ 15).4 The subscore corresponding only to the aforementioned clinical part (i.e., symptoms severity) is reported as the CMS clinical score.
During the inclusion visit, capillary [Hb] was measured using a HemoCue system (Hb201+, HemoCue AB, Ängelholm, Sweden) and transcutaneous oxygen saturation (SpO2) was measured by a finger sensor (Nellcor OxiMax N-65, Medtronic, Dublin, Ireland) after a 5-minute rest period in a sitting position. To avoid inclusion of highlanders with undiagnosed respiratory disease, pulmonary function was assessed during the inclusion visit using a portable spirometer (EasyOne, NDD Medizintechnik AG, Zurich, Switzerland). Forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were reported as percentages of Andean high-altitude population predicted values.21
InterventionAfter completion of the baseline (BL) measurements (see below), participants were randomly assigned to a treatment arm (placebo, acetazolamide 250 mg q.d. or atorvastatin 20 mg q.d.), using a 1:1:1 parallel design, initially for a total treatment duration of 9 months. To avoid potential dose-dependent side-effects that could jeopardize the compliance of the participants, the drug dosages were chosen as the minimal potentially effective dosage.13,18 Both investigators and participants were blinded to the randomization list. Identical capsules of active drugs or placebo were prepared by the pharmacy unit in France and packed in identical box, only identified by the participant ID. Participants were asked to take their capsules every morning, without any supervision. Delivery of the capsule boxes to the participants was organized on a regular basis by a local researcher to check compliance and any side effect.
Outcomes and measurementsAccording to the initially planned study which aimed to assess the long-term (9 months) effects of the treatments, the primary outcome of this study was the change in Hct from BL measurement to 3-week (W3) reassessment. Secondary outcomes at + 3 weeks included the changes in CMS scores, arterial blood gasses, changes in Hbmass and intravascular volumes, changes in erythropoiesis biomarkers, as well as potential adverse effects induced by treatments.
Hematocrit and [Hb] measurements. From a venous heparinized blood sample, Hct was measured using microcentrifuge method (HemataSTAT II, Separation Technology Inc., Sandford, USA) and [Hb] was measured using a co-oximeter (ABL 80 OSM, Radiometer, Copenhagen, Denmark). Mean corpuscular hemoglobin concentration (MCHC) was calculated as [Hb]/Hct ratio.
Arterial blood gasses. An arterial blood sample was obtained from a radial artery puncture, using a pre-heparinized syringe, and was immediately analyzed with a portable blood gas analyzer (i-STAT, Abbott Point of Care, Princeton, New Jersey, USA) to measure arterial pH, arterial partial pressure of oxygen (PaO2, mmHg) and arterial partial pressure of carbon dioxide (PaCO2, mmHg). In addition, serum creatinine and potassium levels were measured to monitor potential diuretic-associated side effects of ACZ.
Total hemoglobin mass and intravascular volumes. Using the carbon monoxide (CO) rebreathing method, Hbmass and derived intravascular volumes [total red blood cell volume (RBCV), PV and total blood volume (BV)] were determined using an automated system (OpCO, Detalo Health, Copenhagen, Denmark).22,23
Erythropoiesis assessment. Serum concentration of erythropoietin (EPO) and soluble transferrin receptor (sTfR), a biomarker of the erythropoietic activity,24 were later measured from serum samples obtained from venous blood after centrifugation and stored at −20 °C until further analyses.
More details about the procedures can be found in the online supplementary material.
Statistical analysisInitial sample size calculation. We estimated that 15 participants per arm would be necessary to detect a within group difference in Hct of 5%, with a power of 80% and a type I error of 5%. This computation was based on Hct values of 74 ± 6% previously observed in CMS inhabitants of La Rinconada22,25 and the 5%-effect of ACZ on Hct previously demonstrated, albeit at lower altitude, for treatment durations ranging from 3 to 24 weeks.13–15 To account for a potential high rate of dropouts at follow-up, we planned to include a total of 60 CMS participants (i.e., 20 participants per arm).
Statistical analysis. After confirming the normality of the main data (using Shapiro-Wilk test and quantile-quantile plots), continuous data were expressed as mean ± standard deviation (SD) or expressed in median [25th-75th percentiles], as appropriate. Categorial data, expressed in absolute count and percentage (%), were compared using Yate's corrected Chi-Squared test or Fisher's exact test. Baseline characteristics between the treatments’ groups were compared using a one-way analyze of variance (ANOVA) or a Kruskal-Wallis test for non-parametric data. Changes over the time of treatment in the three groups were first analyzed using 2 × 2 mixed ANOVA, followed by post-hoc paired t-tests with Bonferroni correction to highlight within-group changes over the time. The non-normally distributed EPO data were log-transformed to meet normality assumption. Within-group percentage changes were provided with their 95% confidence interval (CI). Strength of association between within groups percent changes of relevant variables were performed using Spearman correlation coefficients.
All tests were two-sided and a p-value <0.05 (or a 95%CI excluding zero) was considered significant. All statistical analyses were performed using R software (version 4.1.2) and GraphPad Prism (version 9.3.1).
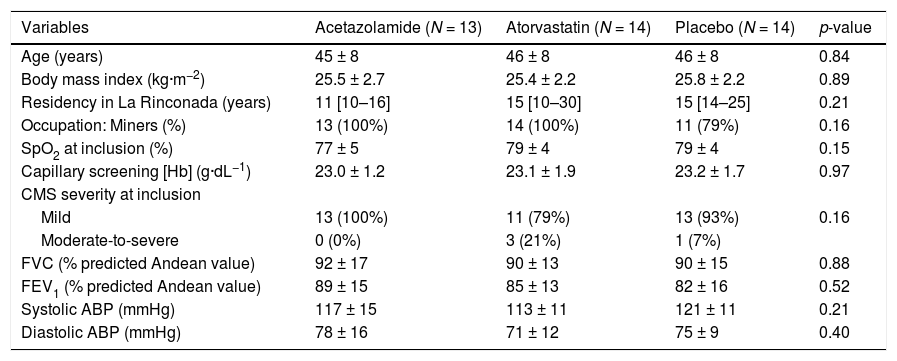
ResultsStudy populationAmong the 60 CMS highlanders initially randomized, 41 (68%) were reevaluated after 19 ± 2 days of treatment in La Rinconada (eFig. 1) and did not differ at baseline from those included but not re-evaluated (eTable 1). Causes of no reassessment included: late inclusion not commensurate with the global expedition schedule (i.e. less than 3 weeks of treatment before the departure of the research team) (n = 9), early loss at follow-up (n = 6) and logistic constraint (n = 4). Baseline characteristics of the 41 patients are provided in Table 1. Most of the patients (n = 37, i.e. 90%) were classified as mild CMS. None of them had chronic medication or declared any other drug intake beyond those provided by the experimenters during the study period.
Baseline characteristics of the 41 chronic mountain sickness highlanders assessed before and after 3 weeks of treatment.
Continuous data are reported in mean ±SD or median [25th-75th percentiles]. Categorial data are reported in absolute count and percentage (%).
SpO2, transcutaneous oxygen saturation; [Hb], hemoglobin concentration; CMS, chronic mountain sickness; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; ABP, arterial blood pressure.
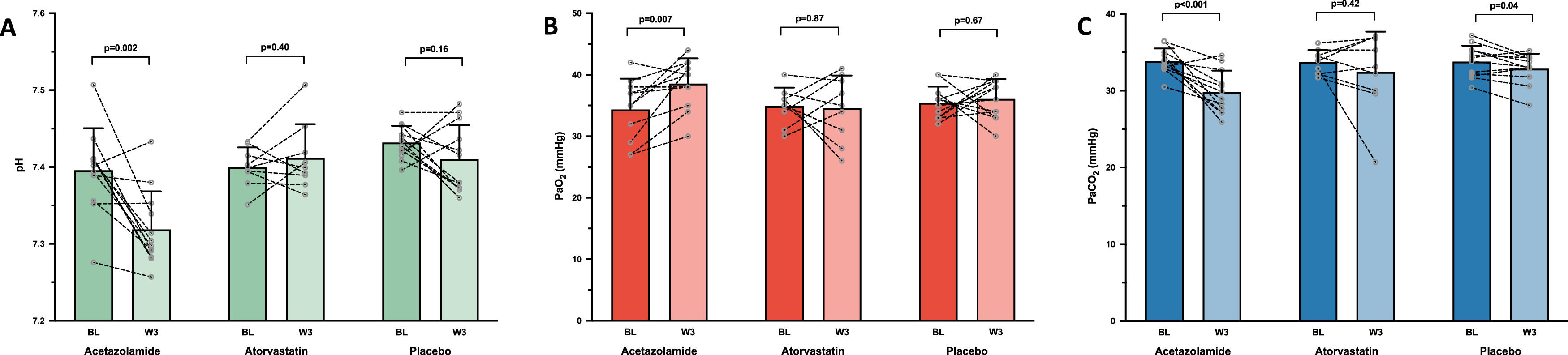
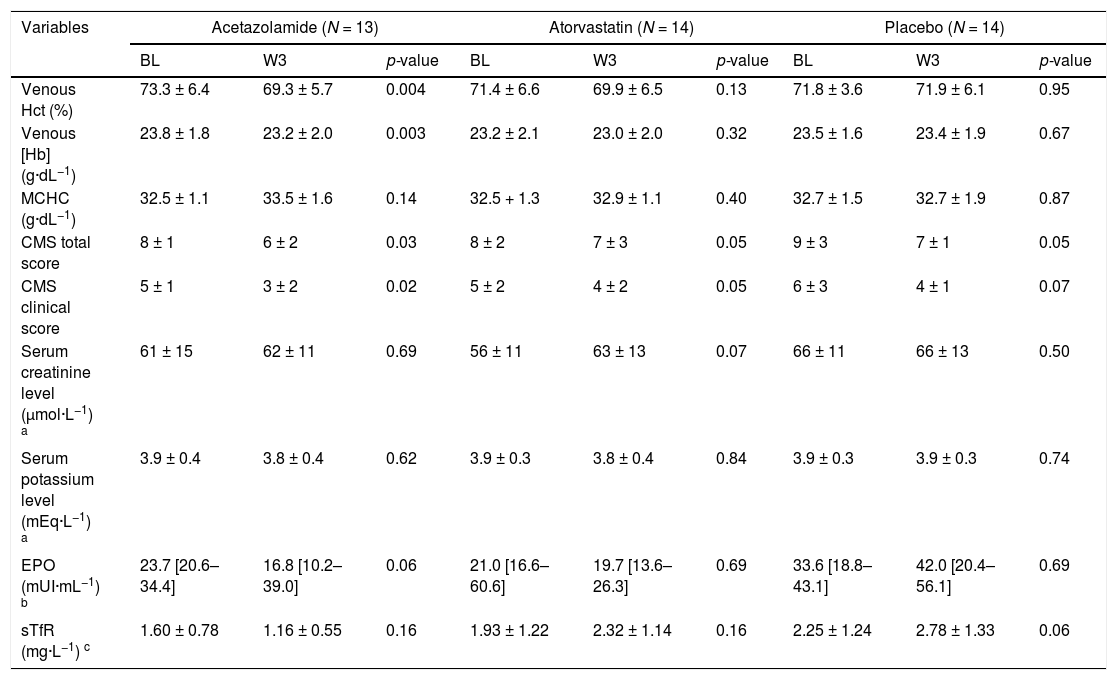
After 3 weeks of drug administration, ACZ significantly reduced Hct and [Hb] by −5.2% (95% CI: −8.3 to −2.2%) and −2.7% (95% CI: −4.4 to −1.1%), respectively, whereas no changes were observed in either the placebo or atorvastatin groups (Table 2). No change in MCHC was observed in any group. CMS severity scores significantly decreased or tended to decrease over the treatment period in all groups (Table 2). ACZ-induced metabolic acidosis (attesting the good observance of patients in the ACZ group) was partially compensated by an increase in ventilation as shown by the significant drop in PaCO2 (Fig. 1A and 1C). This hyperventilation was sufficient to increase PaO2 in the ACZ group (+13.4%, 95% CI: 4.3 to 22.5%) whereas no changes in blood oxygenation were observed in the other two groups (Fig. 1B).
Changes over time (between baseline and 3-week assessment) in primary and secondaries outcomes within the different groups of treatment allocation.
| Variables | Acetazolamide (N = 13) | Atorvastatin (N = 14) | Placebo (N = 14) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BL | W3 | p-value | BL | W3 | p-value | BL | W3 | p-value | |
| Venous Hct (%) | 73.3 ± 6.4 | 69.3 ± 5.7 | 0.004 | 71.4 ± 6.6 | 69.9 ± 6.5 | 0.13 | 71.8 ± 3.6 | 71.9 ± 6.1 | 0.95 |
| Venous [Hb] (g·dL−1) | 23.8 ± 1.8 | 23.2 ± 2.0 | 0.003 | 23.2 ± 2.1 | 23.0 ± 2.0 | 0.32 | 23.5 ± 1.6 | 23.4 ± 1.9 | 0.67 |
| MCHC (g·dL−1) | 32.5 ± 1.1 | 33.5 ± 1.6 | 0.14 | 32.5 + 1.3 | 32.9 ± 1.1 | 0.40 | 32.7 ± 1.5 | 32.7 ± 1.9 | 0.87 |
| CMS total score | 8 ± 1 | 6 ± 2 | 0.03 | 8 ± 2 | 7 ± 3 | 0.05 | 9 ± 3 | 7 ± 1 | 0.05 |
| CMS clinical score | 5 ± 1 | 3 ± 2 | 0.02 | 5 ± 2 | 4 ± 2 | 0.05 | 6 ± 3 | 4 ± 1 | 0.07 |
| Serum creatinine level (µmol·L−1) a | 61 ± 15 | 62 ± 11 | 0.69 | 56 ± 11 | 63 ± 13 | 0.07 | 66 ± 11 | 66 ± 13 | 0.50 |
| Serum potassium level (mEq·L−1) a | 3.9 ± 0.4 | 3.8 ± 0.4 | 0.62 | 3.9 ± 0.3 | 3.8 ± 0.4 | 0.84 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.74 |
| EPO (mUI·mL−1) b | 23.7 [20.6–34.4] | 16.8 [10.2–39.0] | 0.06 | 21.0 [16.6–60.6] | 19.7 [13.6–26.3] | 0.69 | 33.6 [18.8–43.1] | 42.0 [20.4–56.1] | 0.69 |
| sTfR (mg·L−1) c | 1.60 ± 0.78 | 1.16 ± 0.55 | 0.16 | 1.93 ± 1.22 | 2.32 ± 1.14 | 0.16 | 2.25 ± 1.24 | 2.78 ± 1.33 | 0.06 |
Continuous data are reported in mean ±SD) or median [25th-75th percentiles]. Categorial data are reported in absolute count and percentage (%).
BL, baseline; W3, 3 weeks; Hct, hematocrit; [Hb], hemoglobin concentration; MCHC, mean corpuscular hemoglobin concentration; CMS, chronic mountain sickness; EPO, serum concentration of erythropoietin; sTfR, serum concentration of soluble transferrin receptor.
Changes in arterial blood gasses. Mean values with standard deviation and individual variations in arterial pH (A), arterial partial pressure of oxygen [PaO2] (B) and arterial partial pressure of carbon dioxide [PaCO2] (C), measured at baseline (BL) and after 3 weeks of treatment (W3) by acetazolamide (N = 12/13), atorvastatin (N = 9/14) or placebo (N = 12/14), in chronic mountain sickness highlanders permanently living in La Rinconada (5100–5300 m).
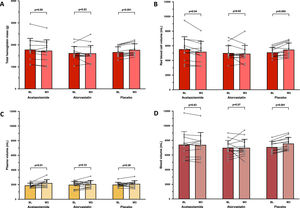
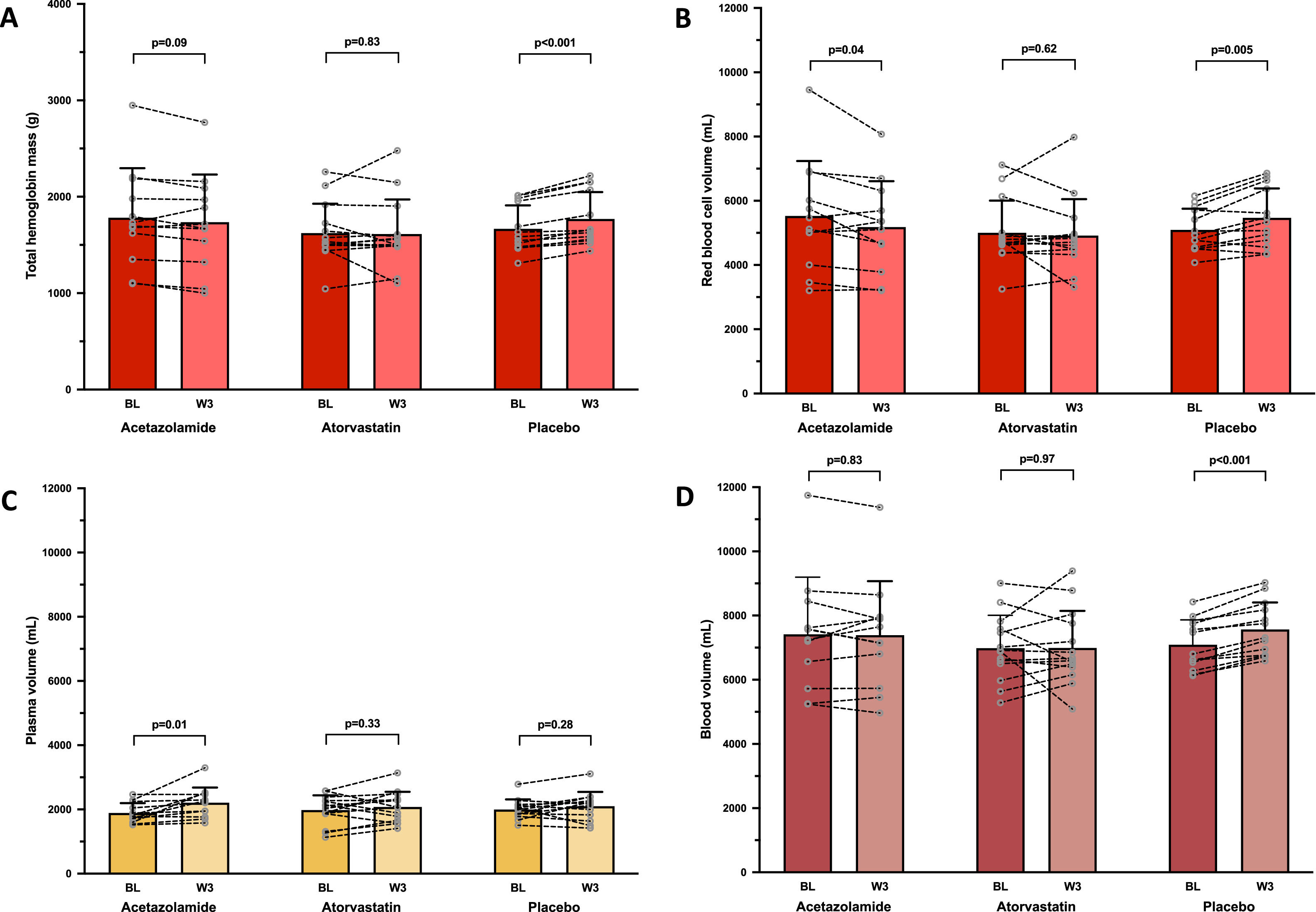
In the ACZ group, Hbmass tended to decrease, albeit non-significantly (−2.6%, 95% CI: −5.7 to 0.5%; Fig. 2A), whereas RBCV significantly decreased (−5.3%, 95% CI: −10.3 to −0.3%; Fig. 2B) and PV significantly increased (+17.6%, 95% CI: 4.9 to 30.3%; Fig. 2C), without any change in BV. EPO level tended to decrease (p = 0.06) in the ACZ group while no significant EPO changes were observed in the two other groups (Table 2).
Changes in intravascular volumes. Mean values with standard deviation and individual variations in total hemoglobin mass (A), red blood cell volume (B), plasma volume (C) and total blood volume (D) measured at baseline (BL) and after 3 weeks of treatment by acetazolamide (N = 12/13), atorvastatin (N = 14/14) or placebo (N = 13/14), in chronic mountain sickness highlanders permanently living in La Rinconada (5100–5300 m).
No changes in Hbmass and in derived intravascular volumes were observed in the atorvastatin group (Fig. 2). An increase in Hbmass was observed in the placebo group (+6.1%, 95% CI: 4.2 to 7.9%; Fig. 2A), as well as in RBCV (+7.0%, 95%CI: 2.7 to 11.4%; Fig. 2B), leading to an elevated BV (+6.7%, 95% CI: 5.0 to 8.4%; Fig. 2D), and a concomitant trend (p = 0.06) towards higher levels of sTfR (Table 1), suggesting a slightly boosted erythropoietic activity.
Correlations in the ACZ groupThe decrease in Hct in the ACZ group was correlated with the increase in PV (ρ=−0.78, p = 0.004) and with the decrease in RBCV (ρ=0.67, p = 0.02); furthermore, the decrease in Hct tended to correlate with the change in EPO (ρ=0.60, p = 0.06, eFig. 2).
The increase in PaO2 in the ACZ group was correlated with the increase in PV (ρ=0.77, p = 0.01) whereas no significant correlation was highlighted between the increase in PaO2 and the change in Hbmass nor in RBCV (eFig. 2).
Drugs toleranceAfter a 3-week period of drug administration, the proportion of participants who complained about specific symptoms such as paresthesia, headache, increased diuresis and muscle or joint soreness did not statistically differ among the groups (data not shown). Furthermore, no significant changes in either serum potassium or in creatinine levels were highlighted during the short-period of treatment (Table 2).
DiscussionThis study assessing the early efficacy and hematological changes induced by drugs potentially improving CMS condition highlights 1) the early efficacy of ACZ to reduce Hct (and [Hb]), even at an extreme altitude > 5000 m and 2) the underlying changes in intravascular volumes. In the ACZ group, the early decrease in Hct observed in the present study was comparable to previous results in highlanders albeit living at an altitude ∼1000 m lower.13–15 As expected, and previously shown,12–15 ACZ administration stimulated ventilation and improved oxygenation, mainly consecutively to a mild metabolic acidosis induced by the inhibition of renal tubular carbonic anhydrase26 and without any identified significant side-effects (e.g., hypokalemia). The relative rise in PaO2 observed in the ACZ group (+13%) was similar to previously observed by others ∼1000 m of altitude lower.15 Despite the absence of any significant change in Hct, decreases in CMS severity scores in the placebo and atorvastatin groups were almost similar than those observed in the ACZ group (Table 2). A similar placebo effect has previously been observed13 and might be, in part, explained by the difficult and subjective evaluation of the symptoms (and their intensity) in CMS population, where the relationship between symptoms’ severity and Hct (or [Hb]) can be inconsistent in cross-sectional studies.22,27,28
In accordance with our hypothesis regarding the effect of ACZ on intravascular volumes in highlanders, the significant increase in PV and the strong correlation between this increase and the decrease in Hct, suggested that the early decrease in Hct was not solely mediated by a decrease in erythrocytosis, but also by fluid regulation leading to an increase in PV, albeit the correlation between Hct and calculated intravascular volumes should be interpreted with caution due to a between-variable dependency. While a decrease in PV could have been expected due to the mild diuretic effect of ACZ,29 this intriguing finding seems in line with previous results observed in a rodent model of chronic hypoxia exposure.16 In this experimental study, ACZ-treated rats during exposure to chronic hypoxia exhibited no significant change in PV compared to normoxic controls, while non-treated hypoxic controls showed the more classically expected hypoxic PV contraction. In lowlanders, reduction in PV is a well-known and rapid physiological response to high-altitude exposure (allowing an increase in arterial oxygen content) with a prompt recovery of PV upon reoxygenation.30 The exact underlying mechanism(s) of the early PV expansion under ACZ therapy observed in our study in CMS highlanders remain(s) to be investigated but may involve, at least in part, an activation of renin-angiotensin-aldosterone system (RAAS) possibly mediated by the oxygenation improvement.16 In Andeans, PV contraction has been shown to contribute, together with an increase in Hbmass, to the CMS polycythemia31,32 and is associated with a decrease in RAAS activity.31 Furthermore, we previously showed that CMS and non-CMS highlanders in La Rinconada had similar Hbmass but that CMS highlanders tended to have a smaller PV, with an inverse relationship between CMS symptoms and PV.22 Taking together, these results support the potential implication of PV in CMS pathophysiology as well as a potential therapeutic target.
The apparent discrepancy between the significant decrease in RBCV (which also correlated with the decrease in Hct) and the non-significant decrease in Hbmass (Fig. 2A and 2B) in the ACZ group could be explained by the small sample size coupled with some inter-individual variability. The more pronounced decrease in RBCV than in Hbmass may also reflect a reduction in reticulocyte and young RBC counts (which have a greater volume than mature and older RBCs33) secondary to the onset of erythropoiesis decline, as suggested by the decreasing trend in plasma EPO level (Table 2). Nevertheless, EPO concentrations must be interpreted cautiously due to a large variability, especially in CMS patients.34 The absence of a complete blood count including reticulocyte count in this study does not allow us to confirm this hypothesis.
At last, the significant increase in Hbmass in the placebo group during this short-term study was unexpected and remains unexplained. Similarly, without any associated measurement of the RASS activity, the absence of concomitant contraction of PV to keep BV constant could not be explained. Participants were well-balanced among the treatment groups and resided at the same altitude (i.e., between 5100 and 5300 m) during the 3-week period. A measurement error seems unlikely for various reasons. First, identical and rigorous CO-rebreathing method was applied at both time-points (see Supplementary Methods) and second, similar pre-measurement fraction of venous carboxyhemoglobin (%HbCO) between the two time-points (eTable 2), allowing us to exclude any drift in %HbCO measurement during the study period. Furthermore, the significant decrease in absolute change in %HbCO during the CO-rebreathing measurement between W3 and BL in the placebo group only (eTable 2) was concordant with the increase in Hbmass, which was also concomitant with the increasing trend in sTfR (Table 2), suggesting a higher erythropoietic activity.24 Although none of subjects included reported any recent prolonged stay at lower altitude and had similar professional activities and life habits, we cannot exclude the fact that some of them may have spent some days at lower altitude (e.g. weekend in Juliaca, 3800 m) or may have been exposed to higher levels of hypoxia during their mining activities. However, the randomized double-blinded study design makes a systematic recruitment bias in one arm unlikely and suggests that differences between groups reflect the effect of the treatment.
Several limitations should be acknowledged. Due to the COVID-19 pandemic and lockdowns, we were not able to proceed with the pre-planned long-term reassessment. As a consequence, we could not determine if a significant Hbmass reduction would occur later in the ACZ or statin groups and we could not evaluate the potential long-term effects of the drugs (especially atorvastatin) on secondary outcomes such as vascular reactivity or oxidative-nitrosative stress.7 The good tolerance and efficacy of ACZ remains to be confirmed over a longer period, even if it has previously been demonstrated up to 6 months albeit at lower altitude.14 Furthermore, mechanisms underlying the early change in PV such as the RAAS activity remain to be elucidated.31 Finally, conducting drug-studies exclusively in male CMS, as in the present study and others,12–15 may restrain the generalizability of results to female CMS, who are also affected by CMS, albeit with a much lower prevalence, at least before menopause.2,3 Studies conducted in female CMS sufferers are unfortunately lacking.
ConclusionsIn summary, 3 weeks of ACZ administration significantly improved oxygenation and reduced Hct in CMS highlanders living at extreme altitude > 5000 m. This early effect on Hct was correlated with both an increase in PV and a decrease in RBCV. Atorvastatin uptake had no effect on hematological parameters after 3 weeks of treatment but longer-term beneficial effects on vascular function may be expected.17 Further studies are required to understand the mechanisms of the ACZ-induced PV increase as well as to determine the role of changes in PV in the adaptative or maladaptive hematological responses to chronic hypoxia in high-altitude dwellers.
Data sharing statementThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Trial registrationClinicalTrials.gov identifier: NCT04251364
Authors’ contributionBC, ES, PR, SD, CAH, AP, AASG, IH, DG, JVB, PC, AP and SV designed the study and/or acquired the data. BC performed the statistical analysis. BC, ES, PR, SD, JVB, PC, AP and SV analyzed and interpreted the data. BC and SV wrote the manuscript; ES, PR, SD, CAH, AP, AASG, IH, DG, JVB, PC and AP revised it critically for important intellectual content. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.



![Changes in arterial blood gasses. Mean values with standard deviation and individual variations in arterial pH (A), arterial partial pressure of oxygen [PaO2] (B) and arterial partial pressure of carbon dioxide [PaCO2] (C), measured at baseline (BL) and after 3 weeks of treatment (W3) by acetazolamide (N = 12/13), atorvastatin (N = 9/14) or placebo (N = 12/14), in chronic mountain sickness highlanders permanently living in La Rinconada (5100–5300 m). Changes in arterial blood gasses. Mean values with standard deviation and individual variations in arterial pH (A), arterial partial pressure of oxygen [PaO2] (B) and arterial partial pressure of carbon dioxide [PaCO2] (C), measured at baseline (BL) and after 3 weeks of treatment (W3) by acetazolamide (N = 12/13), atorvastatin (N = 9/14) or placebo (N = 12/14), in chronic mountain sickness highlanders permanently living in La Rinconada (5100–5300 m).](https://static.elsevier.es/multimedia/25310437/unassign/S2531043723000958/v1_202306011030/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)