The utility of EBUS-TBNA in the diagnosis of sarcoidosis has recently been reported.1,2 Studies published have shown a higher diagnostic yield in favour of EBUS-TBNA, compared to standard bronchoscopic diagnostic techniques, particularly for stage I sarcoidosis.3–6 The main advantages identified, beside the high diagnostic yield, are the low complication rate and being able to avoid invasive procedures, like mediastinoscopy.
Several recent editorials reviewed the value of EBUS-TBNA in excluding other diagnoses in patients with suspected sarcoidosis. In a recent editorial published in the Journal of Bronchology and Intervention Pulmonology, Reich and colleagues estimated that 10,000 patients with stage I sarcoidosis would have to be submitted to an invasive diagnostic procedure to identify, at most, 5 people with an alternative pathology, and questioned the need for tissue confirmation in asymptomatic stage I sarcoidosis.7
Among the series published with patients in suspected stage I and II sarcoidosis that have undergone EBUS-TBNA, only 10% obtained an alternative diagnosis.8 However, these alternative diagnoses should not be ignored. A delay in this context can be harmful to the patient.
What data exists that is available in relation to these questions?
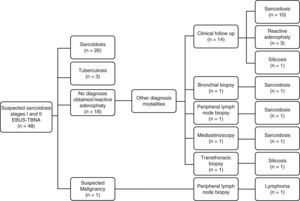
In our experience, 48 patients with clinical and radiological findings suggestive of sarcoidosis underwent EBUS-TBNA (74% stage I and 26% stage II; mean age 45 years). Final diagnosis of sarcoidosis was established in 81% of the patients (39 of 48 patients). The diagnostic yield of EBUS-TBNA for sarcoidosis was 73%, with a sensitivity and specificity of 67% and 100%, respectively. The negative predictive value was 41%. Nine different diagnoses were found: silicosis (n=2), tuberculosis (n=3), non-Hodgkin lymphoma (n=1) and reactive adenopathy (n=3). Thirteen patients received the final diagnosis of sarcoidosis after a negative EBUS-TBNA result. Among them, an additional histological biopsy was only performed in 3 patients (bronchial biopsy, peripheral lymph node biopsy (found after whole-body gallium scan) and surgical lung biopsy). In the remaining 10 patients, 9 had stage I sarcoidosis and 1 had asymptomatic stage II sarcoidosis, the diagnosis was supported by broncoalveolar lavage (CD4+/CD8+ ratio>3.5), radiologic criteria and at least a minimum 6 months follow-up (mean 19±5.7 months) (Figure 1).
Tournoy et al.9 published the results of large diagnostic algorithm implementation trial for sarcoidosis. A total of 137 patients were included (75% stage I). Bronchoscopy was done in 121 patients establishing the definite diagnosis of sarcoidosis in 57 cases (42%). The sensitivity of endoscopic ultrasound following non-diagnostic standard bronchoscopic techniques was 71% and endoscopic ultrasound prevented a surgical procedure in 47 of the 80 patients. The author found that by adding EBUS+EUS to prior nondiagnostic bronchoscopy, three patients had to be investigated in order to avoid one surgical diagnostic procedure. Of the 33 patients left without pathological confirmation, 22 underwent a surgical procedure and an alternative diagnosis was found in only 6 patients. The other 11 patients were submitted to clinical surveillance.
Garwood et al. included1 50 patients with suspected sarcoidosis and a final diagnosis of sarcoidosis was confirmed in 48 patients (one patient was lost to follow-up). The diagnostic yield of EBUS-TBNA was 85% (41 of 48 patients) and was highest in stage I, followed by stage II disease (94% vs. 80% respectively). When EBUS-TBNA result was negative a further histological biopsy was performed in 5 patients (EBUS-targeted TBNA, transbronchial lung biopsy (TBLB) or supraclavicular lymph node biopsy) and 2 were followed up clinically. No patient was submitted to mediastinoscopy and no alternative diagnosis was found.
Wong et al.2 included 65 patients with clinical and radiological findings suggestive of sarcoidosis (74% stage I). The decision to proceed to TBLB was left to the discretion of the operators. In 61 patients the final diagnosis of sarcoidosis was obtained, 56 by EBUS-TBNA and 5 by mediastinoscopy. Three patients with indefinite diagnosis were followed up for ≥18 months and showed no clinical or radiological deterioration. One patient underwent video-assisted thoracoscopic surgery (VATS), after an inadequate EBUS-TBNA specimen, which showed Wegener's granulomatosis.
In the Granuloma clinical trial,10 303 patients with a clinical and radiologic suspicion of sarcoidosis stage I/II (51% stage I) were randomized: 149 to conventional bronchoscopy and 155 to endosonography. The final diagnosis was sarcoidosis in 278 of the 303 patients (92%) which was based on tissue-proven granulomas in 250 of the 278 patients (90%) and in 28 of the 278 patients (10%) on clinical and radiologic follow-up. For stage I sarcoidosis, endosonography had a significantly higher diagnostic yield than bronchoscopy (84% vs. 38%, P<0.001). For stage II sarcoidosis, the difference was not statistically significant (66% vs. 77%, P=0.18). In the bronchoscopy group, biopsies demonstrated eosinophilic and granulomatous vasculitis in one patient and metastasized thyroid cancer in another. In the endosonography group, non-caseating granulomas without necrosis were found in 2 patients, of whom one received a diagnosis of tuberculosis and the other of metastasized non-small cell lung carcinoma. In 2 more patients, a non-small cell lung carcinoma and colon carcinoma nodal metastasis were found.
The EBUS-TBNA increases the diagnostic yield of sarcoidosis (range 71–90% in the studies presented), and reduces the need for more invasive procedures. In every study illustrated above, alternative diagnoses were obtained in a minority of patients. Similar to our results, all authors described clinical follow-up as an alternative to invasive methods after negative EBUS-TBNA,
The role of EBUS-TBNA in the diagnosis of sarcoidosis has become irreplaceable, not only to confirm the diagnosis, but also to exclude other diseases, especially malignancy. Nevertheless, EBUS-TBNA can provide additional difficulties when a differential diagnosis, such as lymphoma, is concerned, and in these cases further invasive procedures can have considerable value.
In our experience, there was no question of performing EBUS-TBNA, since tuberculosis and lymphoma were diagnosed. On the other hand, among negative EBUS-TBNA patients who did not perform additional investigations, no alternative diagnosis emerged.
Nowadays, the great challenge is to know when just EBUS is enough or when to proceed with invasive investigation. What is the correct approach after an EBUS-TBNA negative result?
In our opinion, although looking for granulomatous inflammation is the concern, lymph node sampling by EBUS-TBNA can give us significant information. The appropriate patient selection is the key for the use of EBUS-TBNA in sarcoidosis, and the decision to proceed to further investigation must be based in the pre-test probability of sarcoidosis vs. alternative diagnosis, mainly in stage I sarcoidosis. This approach should be prospectively confirmed.