Cystic fibrosis (CF) is a genetic disease caused by mutations in the CFTR (CF transmembrane conductance regulator) gene1 and characterized by morbidity and mortality primarily related to progressive lung disease.
Over the last decade, modulators correcting CFTR protein folding, processing and trafficking to the cell membrane were developed to improve CFTR function.2
Recently, the triple combination of two CFTR correctors (tezacaftor and elexacaftor) and a CFTR potentiator (ivacaftor) showed exceptional effectiveness in people with CF homozygous for the Phe508del mutation.3,4 A large multicentre phase 3 trial demonstrated clinically significant improvements in lung function within 4 weeks of beginning of elexacaftor–tezacaftor–ivacaftor (ELX/TEZ/IVA) combination and significant improvement in body mass index (BMI), CF quality of life scores and sweat chloride (SwCl) concentration.4
Between February and April 2021, three Phe508del homozygous patients with CF were started on ELX/TEZ/IVA following the approval by European Medicines Agency (EMA). All three were previously on CFTR modulator therapy with lumacaftor–ivacaftor (LUM/IVA), the first combination of a CFTR corrector (lumacaftor) and potentiator (ivacaftor) approved for Phe508del homozygous patients in 2015. The impact of ELX/TEZ/IVA on lung morphology was assessed by chest magnetic resonance imaging (MRI) before and 21–22 weeks after initiation of therapy. MRI T2-weighted sequences and T1-weighted sequences were acquired using a clinical 1.5T MRI scanner (Philips Ingenia; Philips Healthcare, Best, Netherlands) and images were assessed for abnormalities in lung morphology using a dedicated morphology MRI score.5 Data on lung function (i.e. spirometry), nutritional status (BMI), SwCl concentrations and exacerbations before and 24 weeks after the initiation of ELX/TEZ/IVA were also collected from clinical records. In particular, the median forced expiratory volume in the first second (FEV1) and BMI and the mean number of pulmonary exacerbations requiring antibiotics or hospitalization were calculated from the values recorded over the 24 weeks before and after the treatment.
Median age of patients was 17.8 years (range 16.4–35 years), all patients were males and pancreatic insufficient. Median FEV1 was 31.6% predicted (range 68.1–30.6% predicted) and median BMI 20.2 kg·m−2 (range 13.8–21.4 kg·m−2). Baseline mean SwCl concentration was 58.9 ± 14.7 mmol·L−1. Due to pulmonary exacerbations, patients had a mean of 4.6 ± 2 antibiotic courses and a median of 2 hospitalizations (1-4) in the previous 24 weeks before starting ELX/TEZ/IVA.
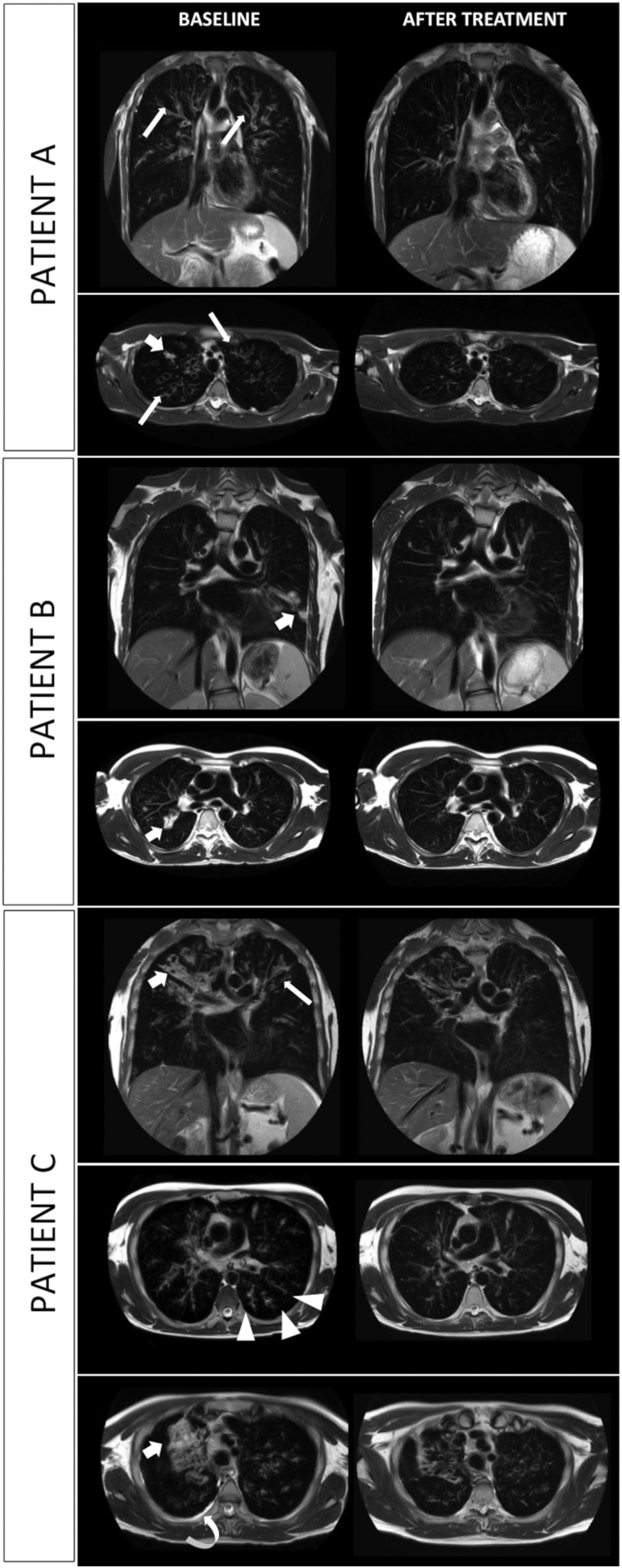
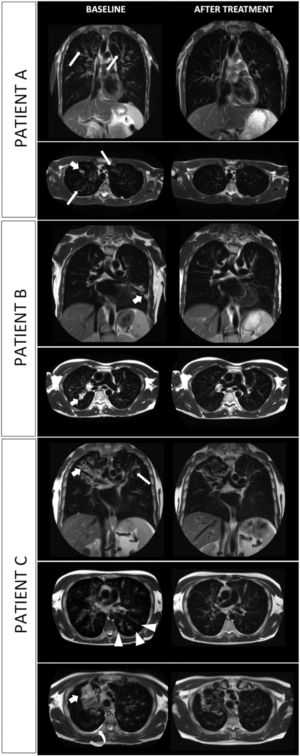
At baseline, MRI showed morphological abnormalities such as bronchiectasis, bronchial wall thickening, mucus plugging and pleural effusion (Fig. 1), resulting in a mean MRI morphology score of 14.7 ± 5.5.
MRI scans of three consecutive patients (A. 18-y-o boy; B. 17-y-o boy; C. 35 y-o man); T2-weighted images on coronal and axial plane are shown. Evolution of most common lung alterations before (left column) and after (right column) initiation of treatment with elexacaftor-tezacaftor-ivacaftor. In every scenario lung consolidation either drastically decreased in extension or completely resolved. Bronchial wall thickening is widely reduced. Tree-in-bud nodules and pleural effusion disappeared (shown in patient C). Bronchiectasis are unchanged in extent and size. Fat arrow: parenchymal consolidation; thin arrow: bronchial wall thickening; arrow-head: tree-in-bud appearance; curved arrow: pleural effusion.
Twenty-four weeks after treatment with ELX/TEX/IVA, median improvement of FEV1 was 15.1% percentage points (range 7.7–36.8%) (median FEV1 post treatment: 67.4% predicted, range 46.7–75.8%). BMI also improved with a mean increase of 1.93 ± 0.95 kg·m−2 (median BMI post treatment 21.8 kg·m−2, range 16.8–22.6 kg·m−2). Mean SwCl was 44.3 ± 21.2 mmol·L−1. Infective exacerbations reduced in frequency with only one exacerbation in one patient requiring a 15-days course of oral antibiotics. ELX/TEX/IVA treatment was generally well tolerated.
After 21–22 weeks of treatment with ELX/TEX/IVA the MRI morphology score was reduced to 4 ± 1 with drastic decrease of lung consolidations and reduction of bronchial wall thickening. Complete resolution of tree-in-bud nodules and pleural effusion was observed in one patient (patient C). In all three cases, bronchiectasis were unchanged in extent and size.
After initiation of ELX/TEX/IVA, Phe508del homozygous patients with CF showed an overall clinical improvement as demonstrated by FEV1, BMI, exacerbation rate and SwCl concentration, and MRI detected an evident favourable evolution in lung structure and morphology.
Interestingly, all patients had been on CFTR modulator therapy with LUM/IVA for a mean of 44 ± 8.5 months. This modulator has been associated with positive effects in terms of lung function, BMI and number of exacerbations6 and with significant changes in lung perfusion and morphology in terms of reduction in pleural reactions7. The further clinical benefits and the improvement in consolidations and airway wall thickening observed in these three patients with the triple combination of ELX/TEZ/IVA highlight the clinical meaning of this novel disease-modifying therapy in patients homozygous for Phe508del.
However, not all people with CF are current candidates for CFTR modulators due to young age or CFTR mutation. Early use of modulators, possibly at time of diagnosis, but also close monitoring and optimal CF care of those waiting for the eligibility are essential to prevent irreversible lung abnormalities.
MRI is a radiation-free imaging technique and a viable alternative to computed tomography. Over the last decade there is increasing evidence that this method can detect subtle changes in lung structure in CF disease. An increasing number of people with CF are now treated with the highly effective therapy of modulators and in these patients MRI may be a sensitive measure to assess early response to treatment and follow-up CF progression.7,8
Ethics StatementThis study was performed in accordance with the Declaration of Helsinki. This human study was approved by Ethic Committee Az. Ospedaliera-Universitaria di Parma (407/2022/OSS/AOUPR). All adult participants provided written informed consent to participate in this study.
CRediT authorship contribution statementV. Fainardi: Conceptualization, Writing – original draft, Data curation, Writing – review & editing, Validation. K. Skenderaj: Formal analysis, Data curation, Investigation, Writing – review & editing, Validation. A. Ciuni: Data curation, Investigation, Formal analysis, Visualization, Writing – review & editing, Validation. S. Esposito: Supervision, Writing – review & editing, Validation. Sverzellati: Supervision, Writing – review & editing, Validation. G. Pisi: Supervision, Writing – review & editing, Validation.