Manual therapy (MT) has been proposed in pulmonary rehabilitation programmes for patients with chronic obstructive pulmonary disease (COPD), but an updated systematic review of the evidence is lacking. We aimed to systematically review the effectiveness of MT interventions, alone or added to exercise, on lung function, exercise capacity and quality of life in COPD patients, compared to other therapies (e.g. exercise alone) or no treatment.
Materials and methodsWe searched MEDLINE, EMBASE, Physiotherapy Evidence Database, and Cochrane Central Register of Controlled Trials databases, using the terms: COPD, manual therapy, manipulation, joint mobilisation, osteopathic manipulation. Only randomised controlled trials (RCT) were considered.
ResultsOut of 555 articles screened, 6 fulfilled the inclusion criteria. The study designs were heterogeneous (with different intervention schedules) and there was a high risk of bias. No effect on lung function was found, while results on exercise capacity were contrasting. MT had no effect on quality of life, although valid measures were available only in one study. Only mild adverse events were reported.
ConclusionsFew RCTs of poor methodological quality are available on the effects of MT in COPD. More and better quality RCTs are needed before this technique can be included in rehabilitation programmes for these patients.
Chronic obstructive pulmonary disease (COPD) is characterised by different phenotypes and often associated with multiple comorbidities contributing to higher mortality rates and reduced physical activity (PA).1 It has been shown that for these patients pulmonary rehabilitation programmes (PRP) can improve symptoms, exercise capacity and health-related quality of life (HRQL),2,3 as a consequence, guidelines for management of COPD recommend that PRP including exercise training should be provided for the vast majority of patients.1
Despite recommendations that PRP be mainly focused on exercise training,2 other manoeuvres are used with low level of evidence in some routine settings with the aim of enhancing the effectiveness of PRP.4 Among these manoeuvres manual therapy (MT) has been proposed also in PRP for COPD patients.5 Manual Therapy has been defined as a clinical approach utilising skilled, specific hands-on techniques, and is used to treat musculoskeletal, soft tissues and joint dysfunctions, with the aim of improving function, modulating pain, and facilitatin movement.5–7 The rationale for proposing MT is that there is a high incidence of musculoskeletal disorders,8 such as cervical and low back pain, and migraine9 in COPD patients, which may benefit from manual interventions. Musculoskeletal disorders and pain have also been mentioned among the causes of impaired physical performance and reduced physical activity in COPD.10
However, there is the need to establish the evidence of effectiveness for MT. A systematic review5 including only techniques used by the physical therapist and papers published before 19909–13 did not find any evidence of effectiveness. Therefore, the aim of this study was to provide a systematic review of randomised controlled trials (RCTs), in order to investigate the effectiveness of MT interventions (alone or added to exercise training), performed by any professional, on lung function, exercise capacity and HRQL in COPD patients. As a secondary aim, we investigated the reported adverse events.
Materials and methodsIn this systematic review of RCTs we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.14 For the purposes of this review, MT was defined as “a clinical approach utilising skilled, specific hands-on techniques” used to “diagnose and treat soft tissues and joint structures for the purpose of modulating pain, increasing range of motion, reducing or eliminating soft tissue inflammation, inducing relaxation, improving contractile and non-contractile tissue repair, extensibility and/or stability, facilitating movement, and improving function”.5
Data sources and search strategiesThe search was conducted from March to September 2017. The following databases were analysed: Medical Literature Analysis and Retrieval System Online (MEDLINE - PubMed), Excerpta Medica dataBASE (EMBASE), Physiotherapy Evidence Database (PEDro) and Cochrane Central Register of Controlled Trials (CENTRAL), from their inception to September 2017. The references of retrieved articles were reviewed for additional studies. The following search terms or medical subject headings (MeSH) were used:
- •
Chronic obstructive pulmonary disease, chronic obstructive airway disease, chronic obstructive lung disease.
- •
Manual therapy, manipulation, musculoskeletal manipulation, joint mobilisation, chiropractic, chiropractic manipulation, osteopathic manipulation techniques and stretching.
The main investigator (M.Vig.) conducted the search of the bibliographic databases, screening all titles and/or abstracts for the inclusion criteria, and identified and removed duplicates. Titles and abstracts of studies were screened by the main investigator to exclude non-eligible studies. Full-text articles were retrieved where the abstract suggested a potentially eligible study. When details were missing from the abstract, full-texts were retrieved and checked for eligibility. Two independent reviewers (M.Vig., C.S.) assessed full-texts for eligibility. Discrepancies were resolved by discussion. The main investigator maintained records on all studies not meeting inclusion criteria, providing the rationale for their exclusion.
Eligibility criteriaWe included RCTs with full-text in English language, according to the following criteria:
Participants: adults with COPD, without any age or disease severity restriction. Studies with mixed population (e.g. combined asthma and COPD) were excluded.
Interventions: RCTs evaluating any form of MT as defined above5 were eligible. Studies were excluded if MT was not delivered through physical hand contact (e.g. by mechanical tools or devices) or consisted only of treatment with complementary and alternative medicine such as point application, acupressure, acupuncture, reflexology, Chinese herbal medicine, tai-chi, yoga or other traditional Chinese medicine techniques. In addition, studies based on MT interventions consisting uniquely of gentle massage, passive stretching, manual chest physiotherapy (such as chest percussion or vibration), and secretion clearance techniques (such as postural drainage or abdominal thrust) were also excluded. Studies assessing patients during an acute exacerbation were also excluded. No restriction on the professional who carried out the MT was placed.
Comparisons: all RCTs comparing MT to usual care, to sham techniques or light manual interventions (e.g. gentle massage), to PA, exercise alone, or to a combinations of these interventions, were included.
Outcome measures: RCTs assessing any of the following outcome measures: lung function [at least forced expiratory volume at 1st second (FEV1)], exercise capacity by six-minute walking test (6MWT), and HRQL, were included. Safety of the interventions (Adverse events) was also evaluated.
Data collection and items: using a dedicated electronic database with the use of Microsoft Excel software (2010 version, Microsoft, Redmond, WA, US), the main investigator extracted the data and the second reviewer (C.S.) checked and revised the database. All entered data were screened for accuracy. Authors of Papers were not asked for missing data. The information included in the database were: study design, setting, sample size, groups, characteristics of participants, details of interventions such as frequency, intensity, time and/or type (FITT), professionals involved, outcome measures and results. Adverse events were classified as ‘mild’, ‘moderate’ and ‘major’, according to the classification described by Carnes et al.15
Risk of bias within studies: all included RCTs were assessed using the Cochrane Risk of Bias tool.16,17 Overall risk of bias for individual studies (low, unclear, high) was assessed by two independent reviewers (M.Vig., C.S.) according to the Cochrane guidelines.17 Discrepancies had to be solved by consensus with a third reviewer (M.P.).
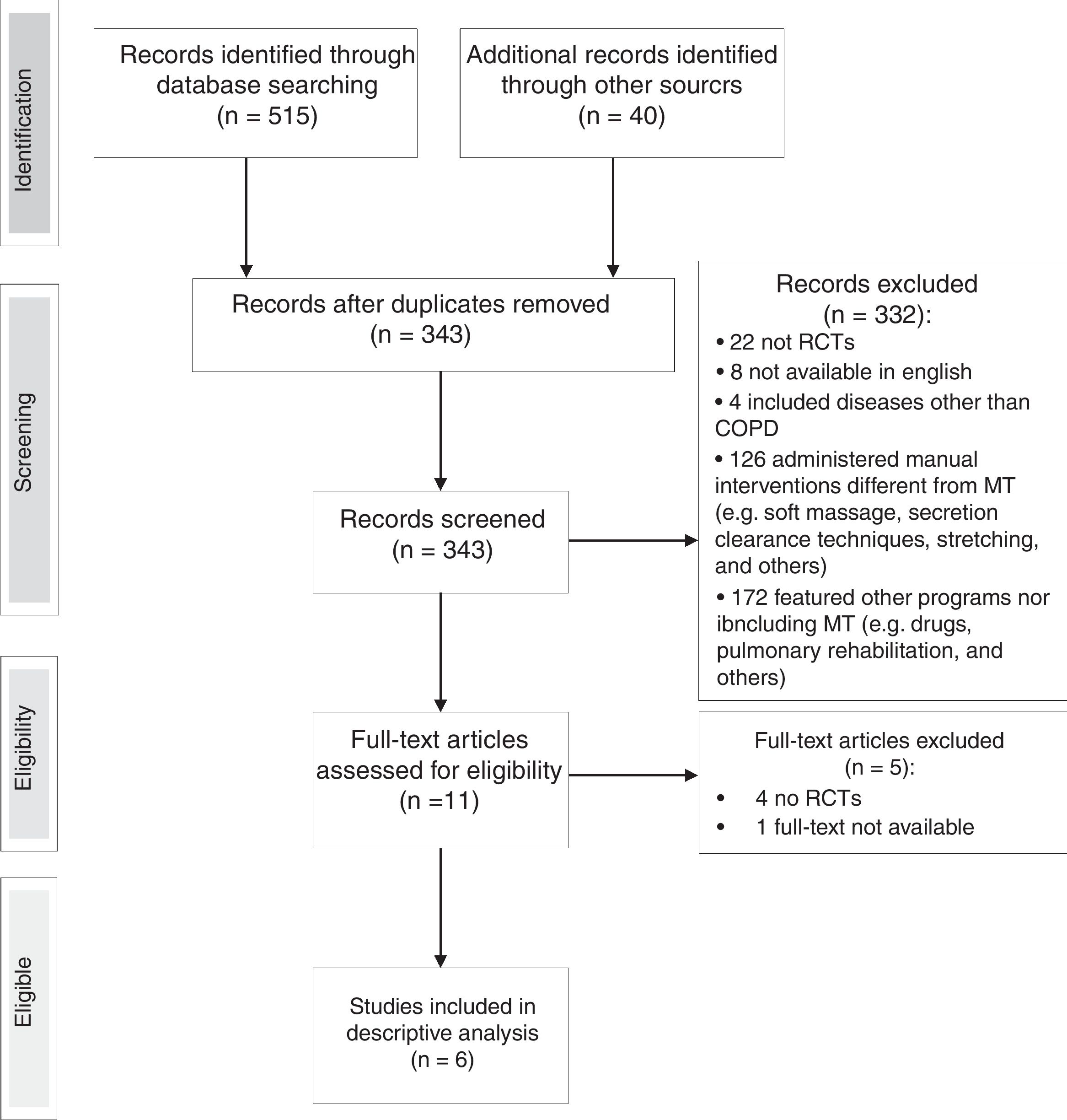
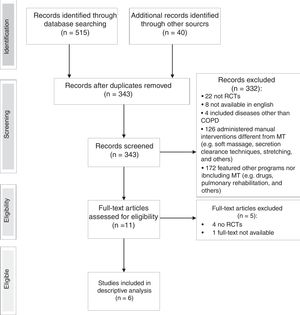
ResultsStudy selectionA flow chart of the selection of studies is shown in Fig. 1. From an initial search of databases, 515 potential studies were identified and another 40 studies were identified from the bibliographies of retrieved articles. After removal of duplicates, 343 titles and abstracts were screened for eligibility, of which 332 were excluded as not meeting the eligibility criteria. Out of the remaining 11, four studies were excluded after review of the full-text, one full-text article was not found. Hence, 6 studies fulfilled the criteria for eligibility and were included in the review. The level of agreement between the two main reviewers was always good and the judgement of the third reviewer was not required.
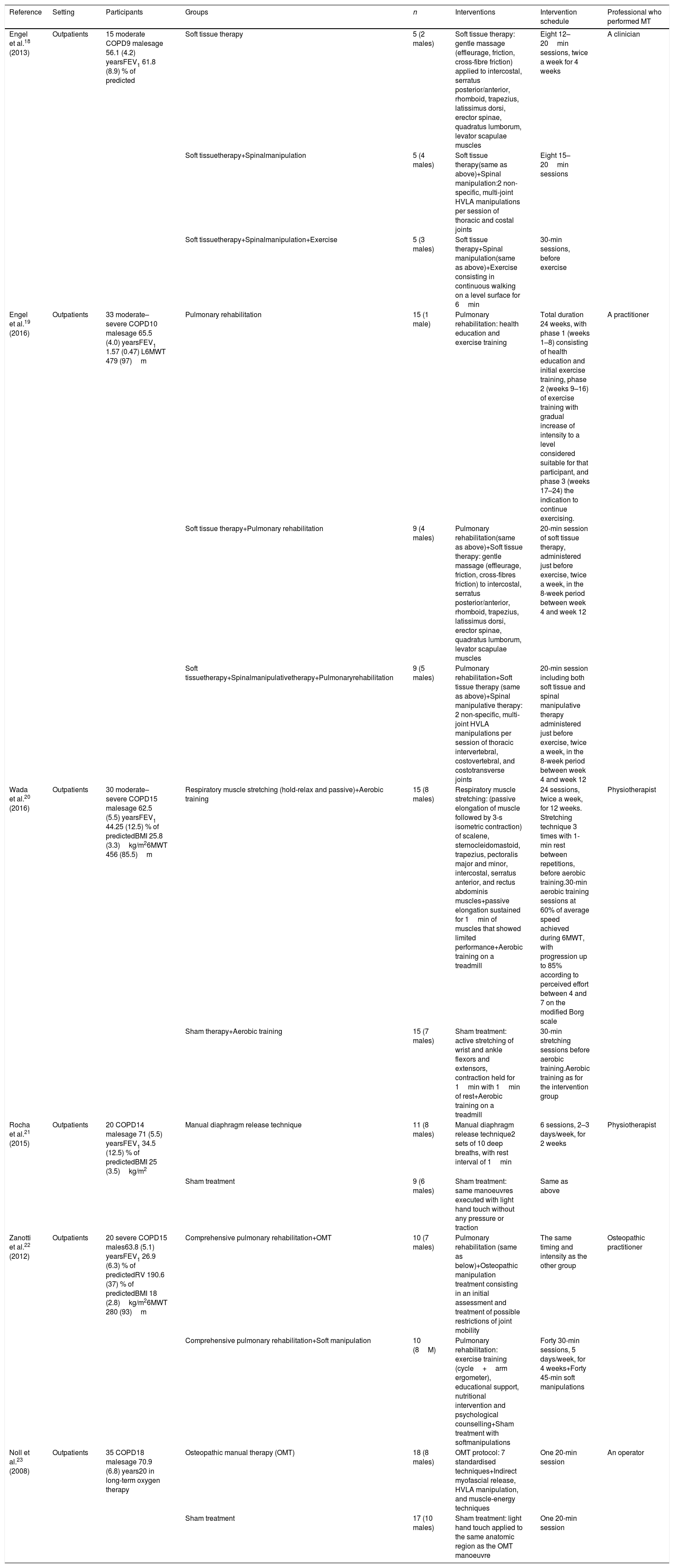
Study characteristicsThe main characteristics of the included RCTs are shown in Table 1. All studies contained a similar hypothesis about the physiological effect of MT: to reduce respiratory muscle hyperactivity and increase the mobility of the thoracic structures involved in respiratory mechanics. All subjects in the included papers were outpatients with moderate to very severe COPD.1 As also shown in Table 1, the intervention schedules varied, consisting of 1–24 sessions lasting 5–45min. As control treatment, all studies18–23 administered sham manoeuvres or a technique that the authors deemed as non-therapeutic, and four RCTs18,22 compared MT also to various exercise programmes or PA.
Characteristics of the included studies.
| Reference | Setting | Participants | Groups | n | Interventions | Intervention schedule | Professional who performed MT |
|---|---|---|---|---|---|---|---|
| Engel et al.18 (2013) | Outpatients | 15 moderate COPD9 malesage 56.1 (4.2) yearsFEV1 61.8 (8.9) % of predicted | Soft tissue therapy | 5 (2 males) | Soft tissue therapy: gentle massage (effleurage, friction, cross-fibre friction) applied to intercostal, serratus posterior/anterior, rhomboid, trapezius, latissimus dorsi, erector spinae, quadratus lumborum, levator scapulae muscles | Eight 12–20min sessions, twice a week for 4 weeks | A clinician |
| Soft tissuetherapy+Spinalmanipulation | 5 (4 males) | Soft tissue therapy(same as above)+Spinal manipulation:2 non-specific, multi-joint HVLA manipulations per session of thoracic and costal joints | Eight 15–20min sessions | ||||
| Soft tissuetherapy+Spinalmanipulation+Exercise | 5 (3 males) | Soft tissue therapy+Spinal manipulation(same as above)+Exercise consisting in continuous walking on a level surface for 6min | 30-min sessions, before exercise | ||||
| Engel et al.19 (2016) | Outpatients | 33 moderate–severe COPD10 malesage 65.5 (4.0) yearsFEV1 1.57 (0.47) L6MWT 479 (97)m | Pulmonary rehabilitation | 15 (1 male) | Pulmonary rehabilitation: health education and exercise training | Total duration 24 weeks, with phase 1 (weeks 1–8) consisting of health education and initial exercise training, phase 2 (weeks 9–16) of exercise training with gradual increase of intensity to a level considered suitable for that participant, and phase 3 (weeks 17–24) the indication to continue exercising. | A practitioner |
| Soft tissue therapy+Pulmonary rehabilitation | 9 (4 males) | Pulmonary rehabilitation(same as above)+Soft tissue therapy: gentle massage (effleurage, friction, cross-fibres friction) to intercostal, serratus posterior/anterior, rhomboid, trapezius, latissimus dorsi, erector spinae, quadratus lumborum, levator scapulae muscles | 20-min session of soft tissue therapy, administered just before exercise, twice a week, in the 8-week period between week 4 and week 12 | ||||
| Soft tissuetherapy+Spinalmanipulativetherapy+Pulmonaryrehabilitation | 9 (5 males) | Pulmonary rehabilitation+Soft tissue therapy (same as above)+Spinal manipulative therapy: 2 non-specific, multi-joint HVLA manipulations per session of thoracic intervertebral, costovertebral, and costotransverse joints | 20-min session including both soft tissue and spinal manipulative therapy administered just before exercise, twice a week, in the 8-week period between week 4 and week 12 | ||||
| Wada et al.20 (2016) | Outpatients | 30 moderate–severe COPD15 malesage 62.5 (5.5) yearsFEV1 44.25 (12.5) % of predictedBMI 25.8 (3.3)kg/m26MWT 456 (85.5)m | Respiratory muscle stretching (hold-relax and passive)+Aerobic training | 15 (8 males) | Respiratory muscle stretching: (passive elongation of muscle followed by 3-s isometric contraction) of scalene, sternocleidomastoid, trapezius, pectoralis major and minor, intercostal, serratus anterior, and rectus abdominis muscles+passive elongation sustained for 1min of muscles that showed limited performance+Aerobic training on a treadmill | 24 sessions, twice a week, for 12 weeks. Stretching technique 3 times with 1-min rest between repetitions, before aerobic training.30-min aerobic training sessions at 60% of average speed achieved during 6MWT, with progression up to 85% according to perceived effort between 4 and 7 on the modified Borg scale | Physiotherapist |
| Sham therapy+Aerobic training | 15 (7 males) | Sham treatment: active stretching of wrist and ankle flexors and extensors, contraction held for 1min with 1min of rest+Aerobic training on a treadmill | 30-min stretching sessions before aerobic training.Aerobic training as for the intervention group | ||||
| Rocha et al.21 (2015) | Outpatients | 20 COPD14 malesage 71 (5.5) yearsFEV1 34.5 (12.5) % of predictedBMI 25 (3.5)kg/m2 | Manual diaphragm release technique | 11 (8 males) | Manual diaphragm release technique2 sets of 10 deep breaths, with rest interval of 1min | 6 sessions, 2–3 days/week, for 2 weeks | Physiotherapist |
| Sham treatment | 9 (6 males) | Sham treatment: same manoeuvres executed with light hand touch without any pressure or traction | Same as above | ||||
| Zanotti et al.22 (2012) | Outpatients | 20 severe COPD15 males63.8 (5.1) yearsFEV1 26.9 (6.3) % of predictedRV 190.6 (37) % of predictedBMI 18 (2.8)kg/m26MWT 280 (93)m | Comprehensive pulmonary rehabilitation+OMT | 10 (7 males) | Pulmonary rehabilitation (same as below)+Osteopathic manipulation treatment consisting in an initial assessment and treatment of possible restrictions of joint mobility | The same timing and intensity as the other group | Osteopathic practitioner |
| Comprehensive pulmonary rehabilitation+Soft manipulation | 10 (8M) | Pulmonary rehabilitation: exercise training (cycle+arm ergometer), educational support, nutritional intervention and psychological counselling+Sham treatment with softmanipulations | Forty 30-min sessions, 5 days/week, for 4 weeks+Forty 45-min soft manipulations | ||||
| Noll et al.23 (2008) | Outpatients | 35 COPD18 malesage 70.9 (6.8) years20 in long-term oxygen therapy | Osteopathic manual therapy (OMT) | 18 (8 males) | OMT protocol: 7 standardised techniques+Indirect myofascial release, HVLA manipulation, and muscle-energy techniques | One 20-min session | An operator |
| Sham treatment | 17 (10 males) | Sham treatment: light hand touch applied to the same anatomic region as the OMT manoeuvre | One 20-min session |
Legend: n, number of patients; COPD, chronic obstructive pulmonary disease; OMT, osteopathic manual therapy; HVLA, high velocity low amplitude; FEV1, forced expiratory volume in 1st sec; RV, residual volume; BMI, body mass index; 6MWT, 6-min walking test.
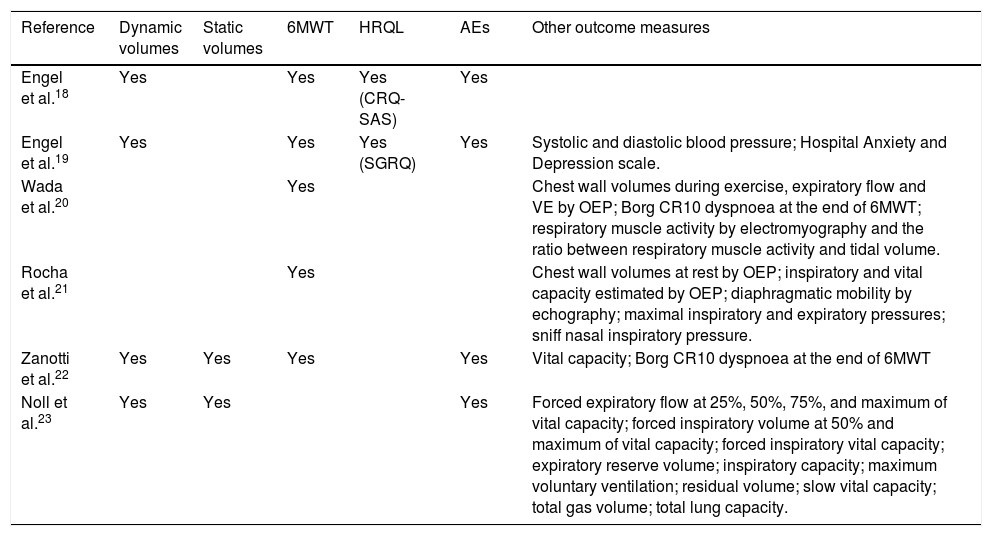
Table 2 shows the outcome measures assessed. Most studies assessed 6MWT and reported adverse effects. Few study assessed lung function.
Summary of outcome measures assessed in the articles reviewed.
| Reference | Dynamic volumes | Static volumes | 6MWT | HRQL | AEs | Other outcome measures |
|---|---|---|---|---|---|---|
| Engel et al.18 | Yes | Yes | Yes (CRQ-SAS) | Yes | ||
| Engel et al.19 | Yes | Yes | Yes (SGRQ) | Yes | Systolic and diastolic blood pressure; Hospital Anxiety and Depression scale. | |
| Wada et al.20 | Yes | Chest wall volumes during exercise, expiratory flow and VE by OEP; Borg CR10 dyspnoea at the end of 6MWT; respiratory muscle activity by electromyography and the ratio between respiratory muscle activity and tidal volume. | ||||
| Rocha et al.21 | Yes | Chest wall volumes at rest by OEP; inspiratory and vital capacity estimated by OEP; diaphragmatic mobility by echography; maximal inspiratory and expiratory pressures; sniff nasal inspiratory pressure. | ||||
| Zanotti et al.22 | Yes | Yes | Yes | Yes | Vital capacity; Borg CR10 dyspnoea at the end of 6MWT | |
| Noll et al.23 | Yes | Yes | Yes | Forced expiratory flow at 25%, 50%, 75%, and maximum of vital capacity; forced inspiratory volume at 50% and maximum of vital capacity; forced inspiratory vital capacity; expiratory reserve volume; inspiratory capacity; maximum voluntary ventilation; residual volume; slow vital capacity; total gas volume; total lung capacity. |
Legend: 6MWT, 6-min walking test; AEs, adverse events; CRQ-SAS, Chronic Respiratory Questionnaire – Self-Administered Standardised; SGRQ, Saint George's Respiratory Questionnaire; VE, minute ventilation; OEP, optoelectronic plethysmography.
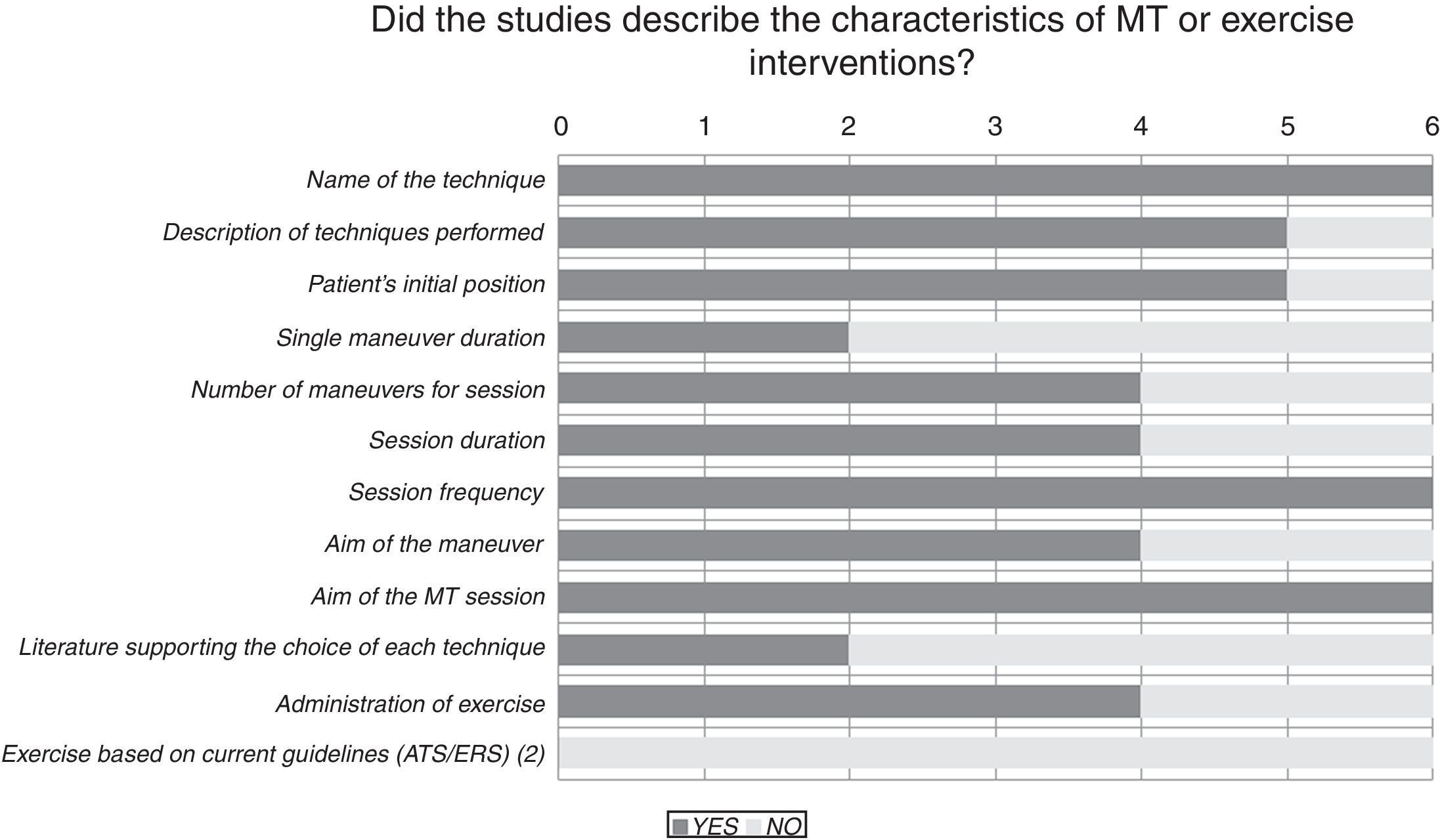
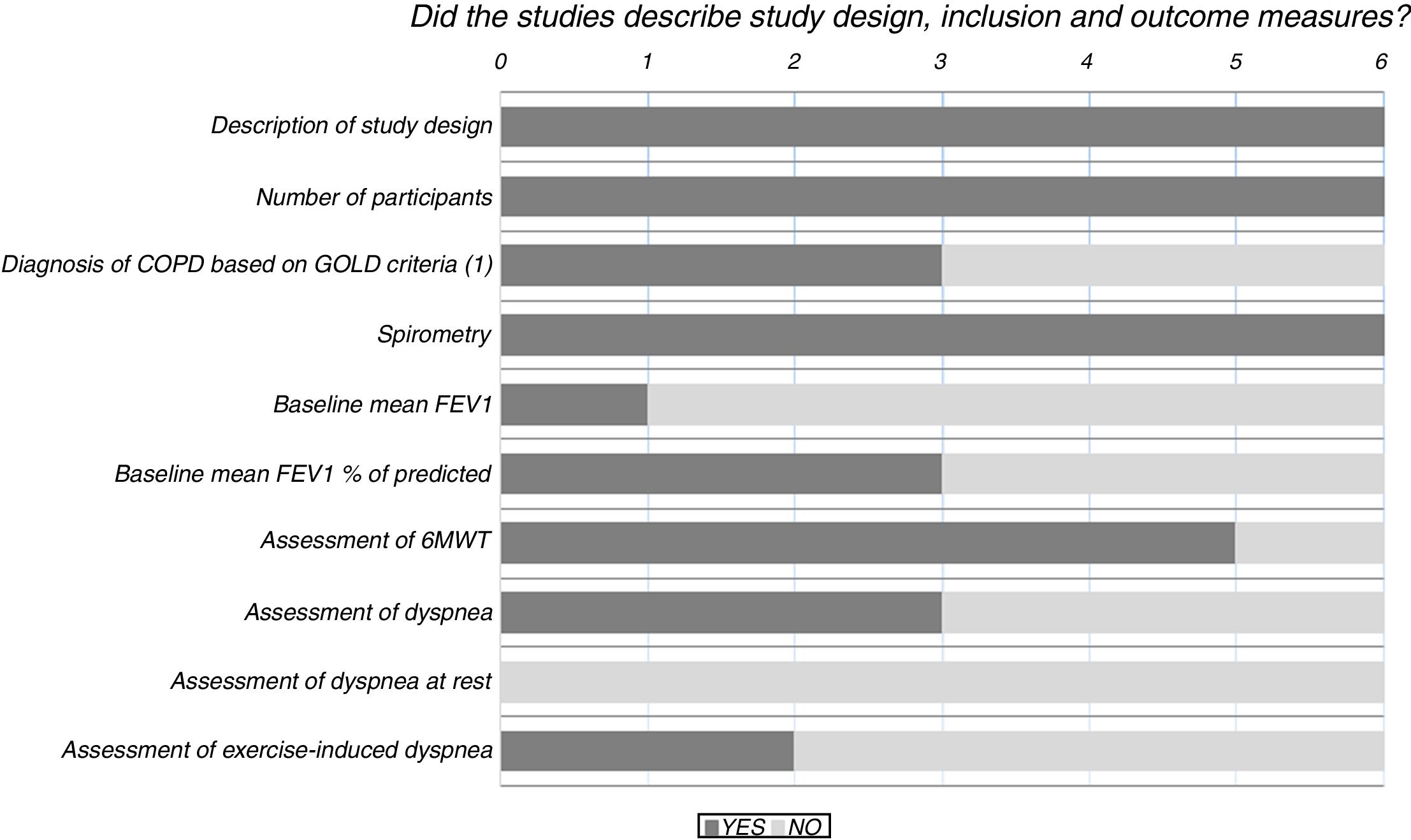
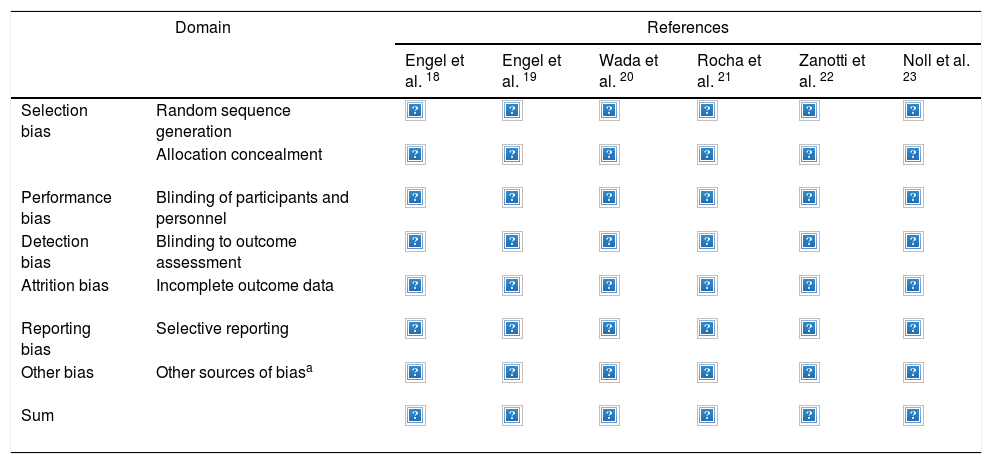
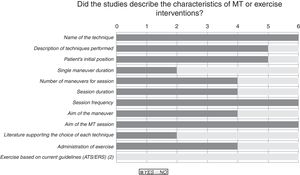
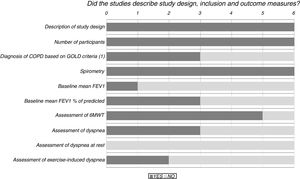
Results of the risk of bias analysis are reported in Table 3. All studies were classified as having a high risk of bias. In one study,18 patients were selected from volunteers recruited through advertisements and newspaper ads, while in another paper19 a pulmonologist selected patients for the study and referred them to the medical centre where they were then randomised and treated. Fig. 2 provides details of the heterogeneity of the characteristics of the interventions: some studies did not state the duration of the sessions,20,21 or did not describe the technique.23Fig. 3 shows the heterogeneity of study designs, inclusion criteria and outcome measures among the included studies. Study designs and sample sizes were described properly in all studies, COPD was diagnosed according to criteria other than GOLD in two papers19,23 or without any clear criteria in another.18
Risk of bias of included studies based on Cochrane Risk of Bias tool 17
| Domain | References | ||||||
|---|---|---|---|---|---|---|---|
| Engel et al. 18 | Engel et al. 19 | Wada et al. 20 | Rocha et al. 21 | Zanotti et al. 22 | Noll et al. 23 | ||
| Selection bias | Random sequence generation | ||||||
| Allocation concealment | |||||||
| Performance bias | Blinding of participants and personnel | ||||||
| Detection bias | Blinding to outcome assessment | ||||||
| Attrition bias | Incomplete outcome data | ||||||
| Reporting bias | Selective reporting | ||||||
| Other bias | Other sources of biasa | ||||||
| Sum | |||||||
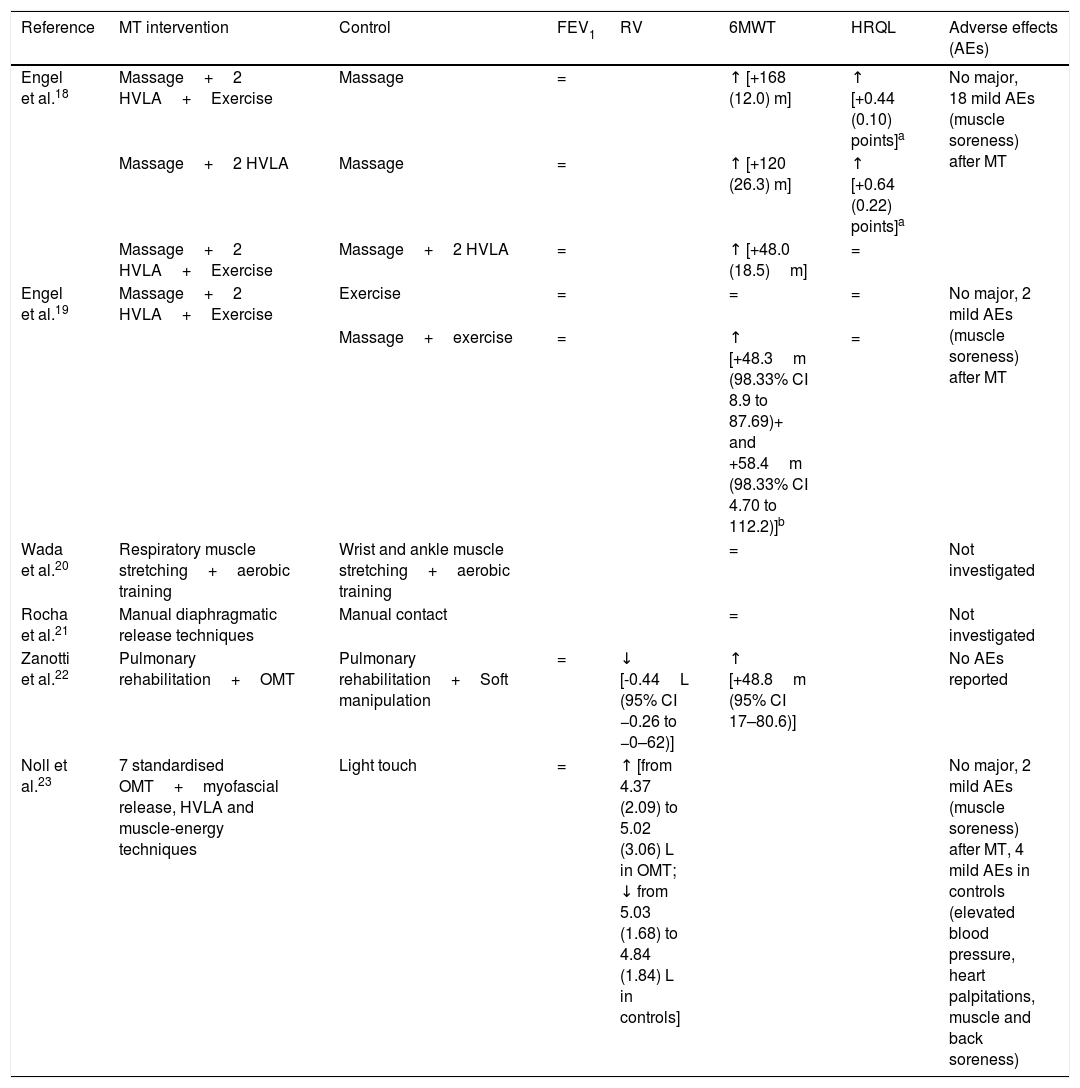
Due to the heterogeneity of the included studies, it was not appropriate to conduct a meta-analysis of the results.
A summary of the main results is shown in Table 4. Noll et al.23 investigated the immediate effects after a single manoeuvre, while all the other studies18–22 investigated the effect of a comprehensive programme. No significant effect on FEV1 was found. Analysing exercise capacity, a significant increase in 6MWT was found in three studies.18,19,22 In the studies18,19 assessing HRQL as an outcome measure, an improvement was reported only by Engel et al.18 who found a reduction in the dyspnoea domain alone. Concerning adverse events, Zanotti et al.22 reported no adverse events or side-effects from osteopathic manipulative therapy, while three studies18,19,23 reported mild adverse events after MT (muscle soreness) in 15% and 0.005% of MT sessions in the two studies by Engel et al.,18,19 respectively, and in two patients out of 35 in the study by Noll et al.23
Synthesis of results of outcome measures.
| Reference | MT intervention | Control | FEV1 | RV | 6MWT | HRQL | Adverse effects (AEs) |
|---|---|---|---|---|---|---|---|
| Engel et al.18 | Massage+2 HVLA+Exercise | Massage | = | ↑ [+168 (12.0) m] | ↑ [+0.44 (0.10) points]a | No major, 18 mild AEs (muscle soreness) after MT | |
| Massage+2 HVLA | Massage | = | ↑ [+120 (26.3) m] | ↑ [+0.64 (0.22) points]a | |||
| Massage+2 HVLA+Exercise | Massage+2 HVLA | = | ↑ [+48.0 (18.5)m] | = | |||
| Engel et al.19 | Massage+2 HVLA+Exercise | Exercise | = | = | = | No major, 2 mild AEs (muscle soreness) after MT | |
| Massage+exercise | = | ↑ [+48.3m (98.33% CI 8.9 to 87.69)+ and +58.4m (98.33% CI 4.70 to 112.2)]b | = | ||||
| Wada et al.20 | Respiratory muscle stretching+aerobic training | Wrist and ankle muscle stretching+aerobic training | = | Not investigated | |||
| Rocha et al.21 | Manual diaphragmatic release techniques | Manual contact | = | Not investigated | |||
| Zanotti et al.22 | Pulmonary rehabilitation+OMT | Pulmonary rehabilitation+Soft manipulation | = | ↓ [-0.44L (95% CI −0.26 to −0–62)] | ↑ [+48.8m (95% CI 17–80.6)] | No AEs reported | |
| Noll et al.23 | 7 standardised OMT+myofascial release, HVLA and muscle-energy techniques | Light touch | = | ↑ [from 4.37 (2.09) to 5.02 (3.06) L in OMT; ↓ from 5.03 (1.68) to 4.84 (1.84) L in controls] | No major, 2 mild AEs (muscle soreness) after MT, 4 mild AEs in controls (elevated blood pressure, heart palpitations, muscle and back soreness) |
Note: aChronic Respiratory Questionnaire – Self-Administered Standardised, domain Dyspnoea; bFVC and 6MWT were collected at a 24-week follow-up, 12 weeks after the conclusion of an 8-week MT intervention; 6MWT was collected at a 16-week follow-up, 4 weeks after the conclusion of an 8-week MT intervention. In brackets are reported the mean improvement of the intervention group (p<0.05) compared to controls.
Legend: ↑, significant increase; ↓, significant decrease; =, no significant change; FEV1, forced expiratory volume at 1st sec; RV, residual volume; 6MWT, 6-min walking test; HRQL, health-related quality of life; AEs, adverse effects; OMT, osteopathic manipulation treatment; HVLA, high velocity low amplitude; CI, confidence interval.
This systematic review investigated the current evidence for effectiveness of MT interventions targeted at musculoskeletal system in COPD patients. The analysis of the six RCTs included18–23 show high heterogeneity of study design, patient sample, MT schedule, and outcome measures assessed. All studies showed a high risk of bias. Interventions were feasible, with only mild adverse events. However, the studies showed no effects on airway obstruction and inconclusive results in static volumes. Inconclusive results were found concerning exercise capacity and only one study showed benefit in HRQL. As a consequence, at present there is no evidence to support the inclusion of MT interventions in standard PRP for patients with COPD.
Our results confirm those by Heneghan et al.,5 which found that pulmonary function change minimally after MT and stated that evidence supporting MT is lacking. Wearing et al.24 instead found that MT, administered in addition to exercise, increased forced vital capacity and functional capacity, but that review24 was focused only on joint manipulation techniques, whereas our review and that by Heneghan et al.5 included a wider variety of techniques. However, that review5 included only MT techniques performed by physical therapists, while we did not limit the search in relation to the professional carrying out MT.
Variability of interventionsThe MT techniques evaluated in the included studies18–23 were either patient- or therapist-dependent. The assumption that a reduction in muscular stiffness and an improvement in joint mobility could produce a COPD-associated decrease of rib cage rigidity was not supported in these studies.18–23 It is unclear whether these manoeuvres may really increase chest wall motion. It has been shown that thoracic high velocity low amplitude techniques improve pain and function in some syndromes such as neck pain,25 shoulder impingement26 and low back pain,27 but effects on chest wall mechanics have not been demonstrated in either healthy or patients with COPD.
The intervention schedules as well as the operator performing them varied among studies. Therefore, it is difficult to compare the interventions, and the contribution of each individual technique to the outcome measures is unknown.
Study qualityThe small sample size of the studies is a serious methodological limitation. It was not clear whether the diagnosis of COPD followed accepted guidelines1 or whether lung function assessments were performed according to standardised methods, which puts into question the comparability of the outcome measures of the studies. No study assessed the specific outcome measures for which MT techniques were aimed at, such as pain. Furthermore, disease-specific HRQL questionnaires were used only in two studies.18,19 Manual therapy was compared to different exercise modalities and schedules but no studies compared MT to a standardised multidisciplinary PRP including high-intensity exercise training.2
No study showed significant effects of MT on airway obstruction. In addition, as a potential adverse effect, in one study23 MT resulted in an immediate increase in static volumes confirming a previous report by the same authors28 but in contrast to another study showing a reduction in static volumes.22 Despite the fact that exercise capacity, as assessed by 6MWT, improved significantly in 3 out of 5 studies18,19,22 the related mechanisms such as a reduction in dyspnoea were assessed only in one study.18 The HRQL did not significantly improve in any study.
LimitationsMissing data were not requested from the authors of papers. Gathering more specific data on the applied technique and on the diagnostic criteria of COPD used, could have reduced the level of bias. Furthermore, the inclusion of RCTs only, and the exclusion of non-English language articles may have induced a publication bias. Another limitation may be due to the fact that various professionals, with different educational paths and different experience, performed MT. Limiting the search to MT manoeuvres performed by specific professionals (e.g. physical therapists) can lead to different results.
Practical implication and future studiesThe present evidence does not support the inclusion of MT in PRP in COPD patients. A standardisation of MT techniques, methods and schedules as well as of outcome measures is necessary to provide a common language, which may be shared across different countries.29 Also the professional carrying out the MT should be always clearly specified. An effort should be made to tailor these techniques to the individual patient,30 and to different COPD phenotypes.31 Future studies should also investigate the effects of MT on symptoms, such as pain, and disability related to musculoskeletal disorders in COPD.
ConclusionsThis systematic review has provided low-quality evidence showing that a variety of MT techniques and programmes, although feasible, have no effects on lung function in COPD patients. Contrasting results have been found regarding the effects on exercise capacity and HRQL. Further studies with better standardisation and scientific validity are needed to assess the effects of MT on exercise capacity, symptoms, disability and quality of life.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
Conflicts of interestThe authors report no conflict of interest.
The Authors thank Rosemary Allpress for the English revision of the manuscript and Laura Comini for editing and technical assistance.