Evidence on effectiveness, safety, and tolerability of imipenem/clavulanate (IC) and linezolid containing regimens to treat multidrug-resistant (MDR-) and extensively drug-resistant tuberculosis (XDR-TB) is scarce. The aim of this observational study is to evaluate the therapeutic contribution of IC and linezolid to manage MDR/XDR-TB cases at the reference centre of São Paulo state, Brazil. Twelve patients (9 males, 1 HIV positive in antiretroviral treatment, 4 MDR, 8 XDR) were treated with IC, 11 of them within linezolid-containing regimens. They all were previously treated with treatment failure, for a median (IQR, interquartile range) of 4.5 (2–6.5) times, having a severe resistance pattern (median number of resistances: 7 (5–8)) and being sputum smear and culture positive. IC and linezolid were prescribed at the dose of 1000mg/day and 600mg/day, respectively. The overall exposure was (median (IQR)) 419 (375.5–658) days for IC and 678 (392–720) days for linezolid. All of them converted their sputum (time to sputum conversion; 60 (37.5–90) days) and culture (75 (60–135) days), and 7 were cured while 5 are still on treatment with a gradually improving clinical picture.
While no adverse events were reported for IC, 2 minor side effects, only, were attributed to linezolid (17%); in both cases the drug was re-started without further problems. Our study suggests that IC and linezolid-containing regimens can be used safely and with satisfactory outcomes in reference centres to treat MDR/XDR-TB patients.
The World Health Organization (WHO) estimated over 480,000 new multidrug-resistant tuberculosis (MDR-TB) cases with 190,000 deaths occurring in 2014. While overall 9.7% of the MDR-TB strains met the criteria defining extensively drug resistant TB (XDR-TB, e.g. resistance to at least one fluoroquinolone and a second line-injectable drug) in some countries of the former Soviet Union this proportion is much higher (29% in Belarus, 15% in Latvia).1–3
Treating MDR- and XDR-TB patients with the drugs available today is known to be long, expensive and complicated, as adverse events (AE) are frequent and often severe.1–4
Presently WHO classifies second-line anti-TB drugs into five groups favouring their stepwise use based on decreasing efficacy and safety from Group 1 to 5. Recent new evidence suggests a revision of the present classification might be necessary.5
Clinicians treating MDR/XDR-TB cases often face difficulties in identifying at least 4 active drugs which are recommended by WHO to compose an effective multi-drug regimen.1–5
Within WHO Group 5, the carbapenems (meropenem, imipenem, ertapenem), are already used to treat MDR/XDR-TB cases, although the evidence available on their efficacy, safety, and tolerability is extremely limited.1–7
Linezolid is also used to treat these cases, being considered effective but often difficult-to-manage because of its frequent and severe AE.8
Evidence on the combined use of carbapenems and linezolid is anecdotal.9
The aim of the present study is to evaluate the potential clinical contribution (effectiveness, safety, and tolerability) of imipenem clavulanate (IC)- and linezolid-containing regimens in treating a cohort of MDR/XDR-TB cases at a referral hospital in Brazil.
Material and methodsThe study, observational and retrospective, has been conducted in the São Paulo state reference centre, Brazil, within a joint project of the European Respiratory Society (ERS) and the Brazilian Thoracic Society. The Centre is served by a quality-controlled laboratory belonging to the WHO network.1 All consecutive MDR-TB cases (TB caused by M. tuberculosis strains phenotypically resistant to at least isoniazid and rifampicin) aged ≥15 years and diagnosed from January 2013 to December 2015 were enrolled.
An individualized TB regimen was administered following the results of the drug-susceptibility test (DST).1
The attending physician prescribed anti-TB drugs without any compelling criteria of experimental protocols and, consequently, blinding or randomized methods were not followed.
IC was administered at a dose of 1000mg 1 time per day plus amoxicillin/clavulanic acid 500/125mg three times a day and linezolid at the dose of 600mg per day.
A standardized ad-hoc e-form was prepared to collect epidemiological (i.e., duration of hospital stay, age, place of birth, sex, residence, immigration from a TB high-burden country), clinical (i.e., HIV status, administration of HIV drugs, previous TB diagnosis and treatment, previous treatment outcomes, radiological findings, TB therapy and related adverse events, duration of exposure to MC and IC, surgery, sputum smear and culture positivity at the treatment baseline, at 30, 60 and 90 days, time to sputum smear and culture conversion, WHO treatment outcomes), and microbiological (i.e., DST results) information from official medical files.
Ethical approval for the collection and analysis of anonymous and retrospective data and for the compassionate use of the drugs is not necessary according to the Brazilian law.
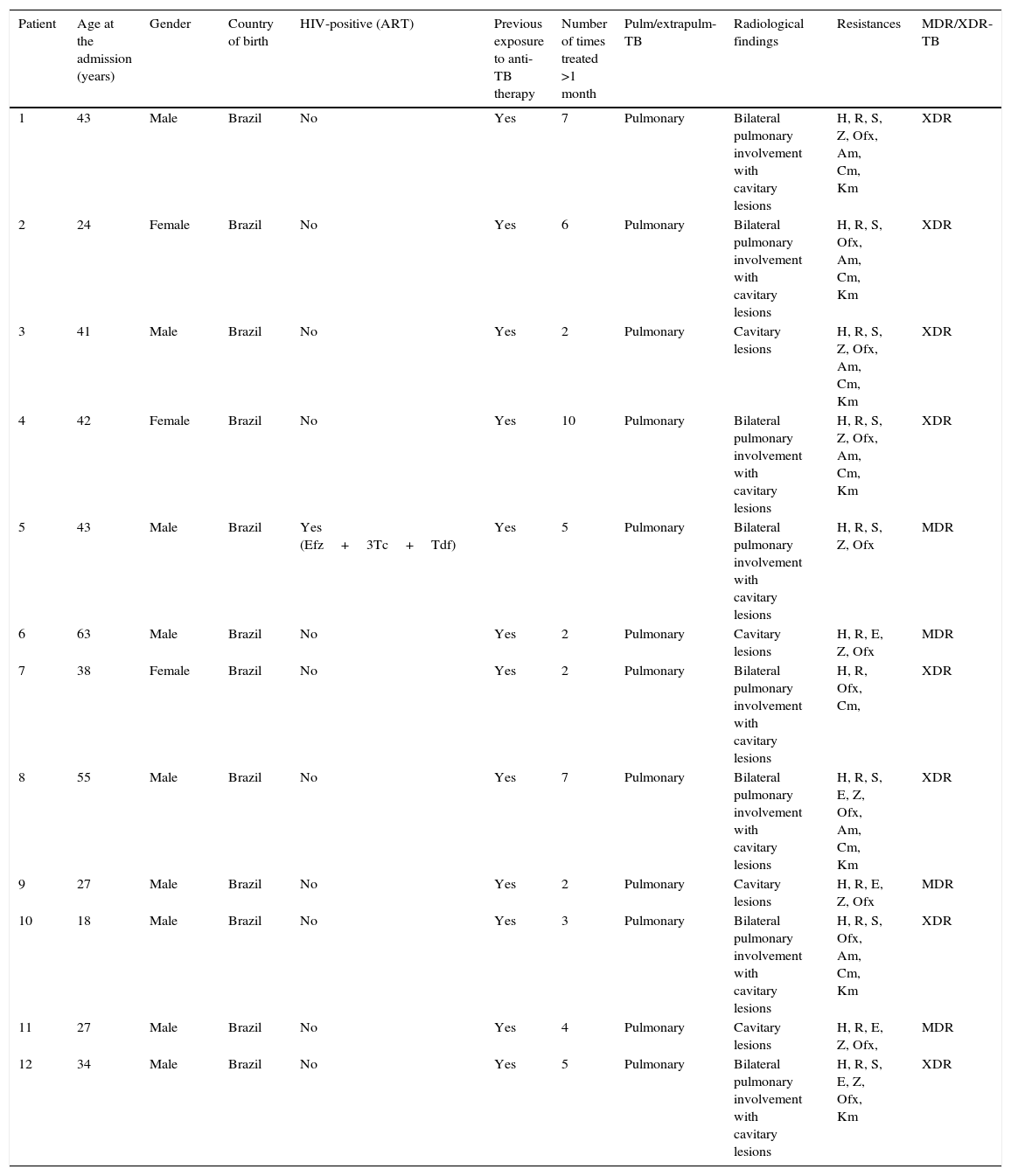
ResultsTwelve patients affected by pulmonary TB were enrolled at a referral hospital in São Paulo state and treated with IC, while 11 of them received also linezolid (Tables 1 and 2).
Demographic and clinical features in the Brazilian cohort.
| Patient | Age at the admission (years) | Gender | Country of birth | HIV-positive (ART) | Previous exposure to anti-TB therapy | Number of times treated >1 month | Pulm/extrapulm-TB | Radiological findings | Resistances | MDR/XDR-TB |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | Male | Brazil | No | Yes | 7 | Pulmonary | Bilateral pulmonary involvement with cavitary lesions | H, R, S, Z, Ofx, Am, Cm, Km | XDR |
| 2 | 24 | Female | Brazil | No | Yes | 6 | Pulmonary | Bilateral pulmonary involvement with cavitary lesions | H, R, S, Ofx, Am, Cm, Km | XDR |
| 3 | 41 | Male | Brazil | No | Yes | 2 | Pulmonary | Cavitary lesions | H, R, S, Z, Ofx, Am, Cm, Km | XDR |
| 4 | 42 | Female | Brazil | No | Yes | 10 | Pulmonary | Bilateral pulmonary involvement with cavitary lesions | H, R, S, Z, Ofx, Am, Cm, Km | XDR |
| 5 | 43 | Male | Brazil | Yes (Efz+3Tc+Tdf) | Yes | 5 | Pulmonary | Bilateral pulmonary involvement with cavitary lesions | H, R, S, Z, Ofx | MDR |
| 6 | 63 | Male | Brazil | No | Yes | 2 | Pulmonary | Cavitary lesions | H, R, E, Z, Ofx | MDR |
| 7 | 38 | Female | Brazil | No | Yes | 2 | Pulmonary | Bilateral pulmonary involvement with cavitary lesions | H, R, Ofx, Cm, | XDR |
| 8 | 55 | Male | Brazil | No | Yes | 7 | Pulmonary | Bilateral pulmonary involvement with cavitary lesions | H, R, S, E, Z, Ofx, Am, Cm, Km | XDR |
| 9 | 27 | Male | Brazil | No | Yes | 2 | Pulmonary | Cavitary lesions | H, R, E, Z, Ofx | MDR |
| 10 | 18 | Male | Brazil | No | Yes | 3 | Pulmonary | Bilateral pulmonary involvement with cavitary lesions | H, R, S, Ofx, Am, Cm, Km | XDR |
| 11 | 27 | Male | Brazil | No | Yes | 4 | Pulmonary | Cavitary lesions | H, R, E, Z, Ofx, | MDR |
| 12 | 34 | Male | Brazil | No | Yes | 5 | Pulmonary | Bilateral pulmonary involvement with cavitary lesions | H, R, S, E, Z, Ofx, Km | XDR |
MDR/XDR-TB: multidrug-resistant/extensively drug-resistant- tuberculosis; ART: anti- retroviral therapy; efz: efavirenz; 3tc: lamivudine; tdf: tenofovir. Drugs: H: isoniazid; R: rifampicin; S: streptomycin; E: ethambutol; Z: pyrazinamide; Am: amikacin; Cm: capreomycin; Ofx: ofloxacin, Km: kanamycin.
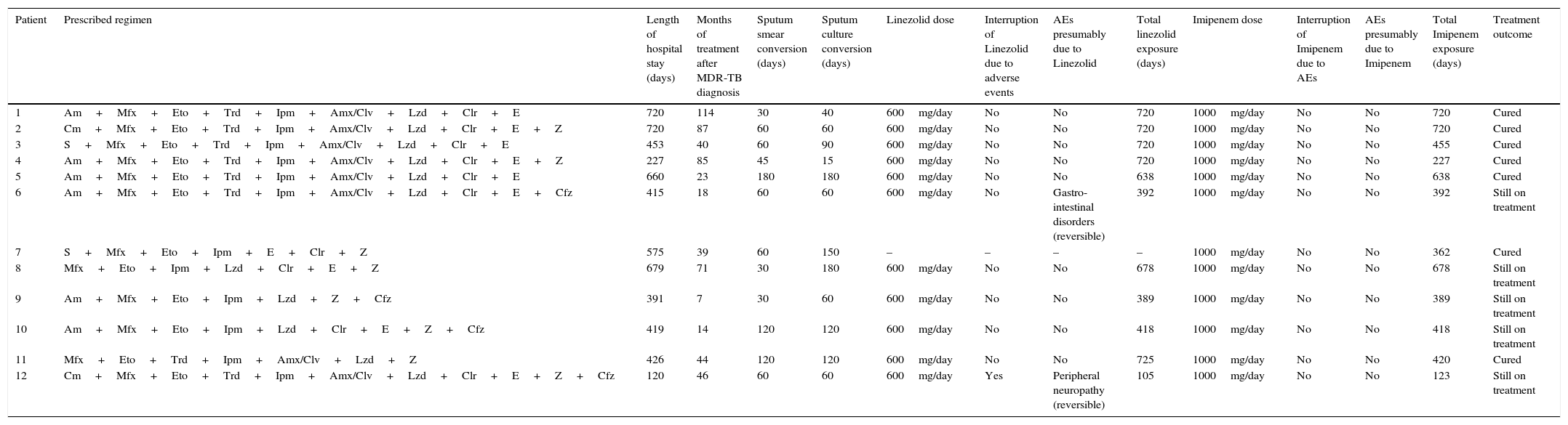
Bacteriology, treatment and outcome in the Brazilian cohort.
| Patient | Prescribed regimen | Length of hospital stay (days) | Months of treatment after MDR-TB diagnosis | Sputum smear conversion (days) | Sputum culture conversion (days) | Linezolid dose | Interruption of Linezolid due to adverse events | AEs presumably due to Linezolid | Total linezolid exposure (days) | Imipenem dose | Interruption of Imipenem due to AEs | AEs presumably due to Imipenem | Total Imipenem exposure (days) | Treatment outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Am+Mfx+Eto+Trd+Ipm+Amx/Clv+Lzd+Clr+E | 720 | 114 | 30 | 40 | 600mg/day | No | No | 720 | 1000mg/day | No | No | 720 | Cured |
| 2 | Cm+Mfx+Eto+Trd+Ipm+Amx/Clv+Lzd+Clr+E+Z | 720 | 87 | 60 | 60 | 600mg/day | No | No | 720 | 1000mg/day | No | No | 720 | Cured |
| 3 | S+Mfx+Eto+Trd+Ipm+Amx/Clv+Lzd+Clr+E | 453 | 40 | 60 | 90 | 600mg/day | No | No | 720 | 1000mg/day | No | No | 455 | Cured |
| 4 | Am+Mfx+Eto+Trd+Ipm+Amx/Clv+Lzd+Clr+E+Z | 227 | 85 | 45 | 15 | 600mg/day | No | No | 720 | 1000mg/day | No | No | 227 | Cured |
| 5 | Am+Mfx+Eto+Trd+Ipm+Amx/Clv+Lzd+Clr+E | 660 | 23 | 180 | 180 | 600mg/day | No | No | 638 | 1000mg/day | No | No | 638 | Cured |
| 6 | Am+Mfx+Eto+Trd+Ipm+Amx/Clv+Lzd+Clr+E+Cfz | 415 | 18 | 60 | 60 | 600mg/day | No | Gastro-intestinal disorders (reversible) | 392 | 1000mg/day | No | No | 392 | Still on treatment |
| 7 | S+Mfx+Eto+Ipm+E+Clr+Z | 575 | 39 | 60 | 150 | – | – | – | – | 1000mg/day | No | No | 362 | Cured |
| 8 | Mfx+Eto+Ipm+Lzd+Clr+E+Z | 679 | 71 | 30 | 180 | 600mg/day | No | No | 678 | 1000mg/day | No | No | 678 | Still on treatment |
| 9 | Am+Mfx+Eto+Ipm+Lzd+Z+Cfz | 391 | 7 | 30 | 60 | 600mg/day | No | No | 389 | 1000mg/day | No | No | 389 | Still on treatment |
| 10 | Am+Mfx+Eto+Ipm+Lzd+Clr+E+Z+Cfz | 419 | 14 | 120 | 120 | 600mg/day | No | No | 418 | 1000mg/day | No | No | 418 | Still on treatment |
| 11 | Mfx+Eto+Trd+Ipm+Amx/Clv+Lzd+Z | 426 | 44 | 120 | 120 | 600mg/day | No | No | 725 | 1000mg/day | No | No | 420 | Cured |
| 12 | Cm+Mfx+Eto+Trd+Ipm+Amx/Clv+Lzd+Clr+E+Z+Cfz | 120 | 46 | 60 | 60 | 600mg/day | Yes | Peripheral neuropathy (reversible) | 105 | 1000mg/day | No | No | 123 | Still on treatment |
AE: adverse event. Drugs: S: streptomycin; Z: pyrazinamide; E: ethambutol; Am: amikacin; Mfx: moxifloxacin; Eto: ethionamide; Trd: terizidone; Ipm: imipenem; Amx/Clv: amoxicilin/clavulanate; Lzd: linezolid; Clr: claritromycin; Cm: capreomycin; Cfz: clofazimine; Ipm: imipenem.
Nine were males (75%) and 3 females, with a median (IQR) age of 39.5 (27–43) years. A single patient was HIV positive, being in regular antiretroviral treatment with a combination of lamivudine, efavirenz and tenofovir, one had diabetes, one hypertension, 2 were admitted with acute respiratory failure, while 7 were drug abusers and 4 alcohol addicts before admission.
Four cases met the definition of MDR-TB and 8 of XDR-TB.
They all were previously treated (treatment failure being the last outcome), for a median of 4.5 (2–6.5) times.
The cases had a severe resistance pattern (median (IQR) number of resistance 7 (5–8)) and were sputum smear and culture positive when referred to the São Paulo State Secretary of Health. All of them had cavities in the chest radiography, being bilateral in 8 cases (67%).
The overall exposure was (median (IQR)) 419 (375.5–658)) days for IC and 678 (392–720) days for linezolid. They required long hospitalization at the reference Centre (439.5 (403–669.5) days).
All of them converted their sputum (time to sputum conversion: 60 (37.5–90) days) and culture (75 (60–135) days), and 7 (58%) were cured while 5 are still on treatment with a gradually improving clinical picture.
While no adverse events were reported for IC, 2 minor and reversible AEs only were attributed to linezolid (17%): peripheral neuropathy in patient 12 (linezolid was re-started without further problems) and gastro-intestinal disorders in patient 6 (diarrhoea, managed with symptomatic medications without need to stop the anti-TB drugs).
DiscussionThis is the first study reporting bacteriological conversion information and treatment outcomes in a Latin American cohort of MDR/XDR-TB cases treated with IC within linezolid-containing regimens. A single patient was prescribed IC but not linezolid to avoid the co-administration with ethionamide due to a prior history of peripheral neuropathy.
The anti-TB regimens have been designed as per WHO guidelines and guided by drug susceptibility testing, taking into account the following: (1) kanamycin was not available in Brazil and capreomycin was available after 2014; (2) pyrazinamide was not used when intolerance to it was documented; (3) terizidone is used in Brazil instead of cycloserine; and (4) PAS (para-aminosalicylic acid) has been made available in Brazil after 2014.
The results of our study demonstrate that, in spite of the cases severity, IC within linezolid containing regimens is able to: (1) ensure sputum smear and culture conversion in all the cases of the cohort, with time to bacteriological conversion similar to that recently described by the International Carbapenems Study Group1–3; (2) reach a positive treatment outcome in 7 out of 12 cases, while the remaining 5 are improving clinically and radiologically and remain consistently sputum smear and culture negative; and (3) is safe, if managed at reference centre level, with two minor and reversible AEs.
Based on its molecular mechanism of action, imipenem is more active than meropenem.1,3 However, this does not necessarily translate into better clinical results, as shown in a recent multinational study.3 Furthermore, the study results confirm the importance of prescribing imipenem in association with clavulanate (a β-lactamase inhibitor) which can inhibit the activity of the potent β-lactamase, encoded by the BlaC gene.1–3
The observational and retrospective design of the study has in-built limitations (impossibility to pre-calculate the sample size, to have a control group, to randomize and ensure blindness).
Additional limitations are the small sample size (with limited inclusion of patients with HIV co-infection) and the fact that these patients received several prior anti-TB drugs. However, the study is, as of today, the third in the literature (the first in Latin America) and this anecdotal evidence might be of help.
In conclusion, the study results confirm that IC, within the carbapenems class of drugs might have a role in treating MDR/XDR-TB and that IC and linezolid-containing regimens can be used safely and with satisfactory outcomes in reference centres to treat MDR/XDR-TB patients.9,10
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Our special thanks to Ms Heloisa Esteves de Souza da Cunha and Sonia Aparecida Telles Torres for data collection at Nestor Goulart Reis Hospital.