Bronchiectasis is a highly complex entity that can be very challenging to investigate and manage. Patients are diverse in their aetiology, symptoms, risk of complications and outcomes. “Endotypes”- subtypes of disease with distinct biological mechanisms, has been proposed as a means of better managing bronchiectasis. This review discusses the emerging field of endotyping in bronchiectasis.
We searched PubMed and Google Scholar for randomized controlled trials (RCT), observational studies, systematic reviews and meta-analysis published from inception until October 2022, using the terms: “bronchiectasis”, “endotypes”, “biomarkers”, “microbiome” and “inflammation”. Exclusion criteria included commentaries and non-English language articles as well as case reports. Duplicate articles between databases were initially identified and appropriately excluded.
Studies identified suggest that it is possible to classify bronchiectasis patients into multiple endotypes deriving from their co-morbidities or underlying causes to complex infective or inflammatory endotypes. Specific biomarkers closely related to a particular endotype might be used to determine response to treatment and prognosis. The most clearly defined examples of endotypes in bronchiectasis are the underlying causes such as immunodeficiency or allergic bronchopulmonary aspergillosis where the underlying causes are clearly related to a specific treatment. The heterogeneity of bronchiectasis extends, however, far beyond aetiology and it is now possible to identify subtypes of disease based on inflammatory mechanisms such airway neutrophil extracellular traps and eosinophilia. In future biomarkers of host response and infection, including the microbiome may be useful to guide treatments and to increase the success of randomized trials.
Advances in the understanding the inflammatory pathways, microbiome, and genetics in bronchiectasis are key to move towards a personalized medicine in bronchiectasis.
Bronchiectasis is a complex and heterogenous disease with multiple aetiologies and comorbidities.1 Differences in the aetiology, epidemiology and microbiology of bronchiectasis can be observed across countries and continents which may influence the pathogenesis in this disease, by different molecular pathways – the endotypes – which converge in airway inflammation and permanent dilation of the bronchi.2,3 Endotypes refer to subtypes of a disease which have distinct biological mechanisms that may link to phenotype, clinical outcomes or treatment response.
Treatment of bronchiectasis primarily consists of airway clearance therapies/techniques and antibiotic therapy, whether for maintenance or during exacerbations.4 Responses to treatment in bronchiectasis are inconsistent as illustrated by the failure of inhaled antibiotics and mucoactive drugs to show benefits in several randomized trials.5,6 Thus, there is an urgent need to better understand the underlying inflammatory and microbiological contributions for the pathogenesis, to better stratify patients in different endotypes prone to target therapies.7 A treatable traits approach, based on the recognition of endotypes, might guide us towards precision medicine and, subsequently, converge in better clinical outcomes.8,9
It is now possible to integrate and analyse extensive novel biological data from patients to identify relevant disease biomarkers and associations - known as “multi-omics” (which includes genomics, transcriptomics, proteomics, metabolomics, lipidomics, and glycosomics).10 Multi-omics is significantly advancing our understanding of the pathophysiology of bronchiectasis and has generated a number of new potential biomarkers.
The objective of this review was to review recent advances in understanding the pathophysiology bronchiectasis (excluding cystic fibrosis) with a specific focus on data identifying subtypes of disease with therapeutic implications, also known as endotypes.
MethodsWe searched PubMed and Google Scholar for randomized controlled trials (RCT), observational studies, systematic reviews and meta-analysis published from inception until October 2022, using the terms: “bronchiectasis”, “endotypes”, “biomarkers”, “microbiome” and “inflammation”. Exclusion criteria included commentaries and non-English language articles as well as case reports. Duplicate articles between databases were initially identified and appropriately excluded.
ResultsThe search process yielded 2131 articles. After a careful analysis of the title and abstract, we included 118 articles. This information was summarized in a narrative review.
The identification of candidate endotypes involves the integration of clinical patient data, underlying cause, microbiology and inflammatory data to classify patients into meaningful subgroups. In considering endotyping in bronchiectasis we will discuss these factors in isolation followed by efforts to understand how they link together to identify candidate subtypes of disease.
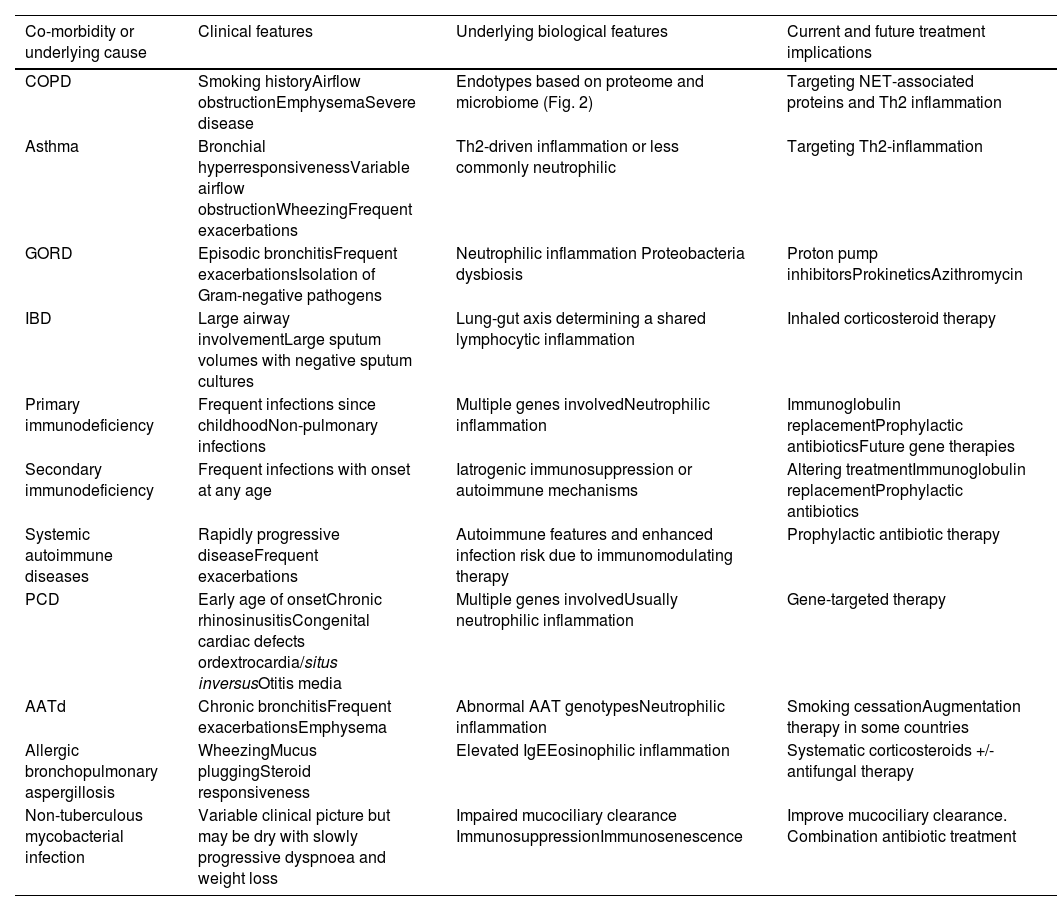
We will first discuss the major co-morbidities and underlying causes of bronchiectasis and what data supports their role within bronchiectasis endotypes (Table 1).
Endotypes in bronchiectasis according to main co-morbidities or underlying causes.
| Co-morbidity or underlying cause | Clinical features | Underlying biological features | Current and future treatment implications |
|---|---|---|---|
| COPD | Smoking historyAirflow obstructionEmphysemaSevere disease | Endotypes based on proteome and microbiome (Fig. 2) | Targeting NET-associated proteins and Th2 inflammation |
| Asthma | Bronchial hyperresponsivenessVariable airflow obstructionWheezingFrequent exacerbations | Th2-driven inflammation or less commonly neutrophilic | Targeting Th2-inflammation |
| GORD | Episodic bronchitisFrequent exacerbationsIsolation of Gram-negative pathogens | Neutrophilic inflammation Proteobacteria dysbiosis | Proton pump inhibitorsProkineticsAzithromycin |
| IBD | Large airway involvementLarge sputum volumes with negative sputum cultures | Lung-gut axis determining a shared lymphocytic inflammation | Inhaled corticosteroid therapy |
| Primary immunodeficiency | Frequent infections since childhoodNon-pulmonary infections | Multiple genes involvedNeutrophilic inflammation | Immunoglobulin replacementProphylactic antibioticsFuture gene therapies |
| Secondary immunodeficiency | Frequent infections with onset at any age | Iatrogenic immunosuppression or autoimmune mechanisms | Altering treatmentImmunoglobulin replacementProphylactic antibiotics |
| Systemic autoimmune diseases | Rapidly progressive diseaseFrequent exacerbations | Autoimmune features and enhanced infection risk due to immunomodulating therapy | Prophylactic antibiotic therapy |
| PCD | Early age of onsetChronic rhinosinusitisCongenital cardiac defects ordextrocardia/situs inversusOtitis media | Multiple genes involvedUsually neutrophilic inflammation | Gene-targeted therapy |
| AATd | Chronic bronchitisFrequent exacerbationsEmphysema | Abnormal AAT genotypesNeutrophilic inflammation | Smoking cessationAugmentation therapy in some countries |
| Allergic bronchopulmonary aspergillosis | WheezingMucus pluggingSteroid responsiveness | Elevated IgEEosinophilic inflammation | Systematic corticosteroids +/- antifungal therapy |
| Non-tuberculous mycobacterial infection | Variable clinical picture but may be dry with slowly progressive dyspnoea and weight loss | Impaired mucociliary clearance ImmunosuppressionImmunosenescence | Improve mucociliary clearance. Combination antibiotic treatment |
Chronic obstructive pulmonary disease (COPD) and bronchiectasis (BE) are two diseases with overlapping clinical presentation, and increased susceptibility to exacerbations. Both entities are defined by different criteria but simultaneous diagnosis still occurs, termed as the COPD-BE association.11 Poorer outcomes have been widely reported but the underlying biological mechanisms leading to those outcomes have not been studied until recently.11
Analysis from sputum microbiome (using 16 s rRNA amplicon sequencing) and protein profiling revealed that patients with the COPD-BE association had a higher abundance of Proteobacteria (microbiome phylum containing the pathogenic Gram-negative organisms such as Pseudomonas aeruginosa), higher expression of the pro-inflammatory mucin MUC5AC, and proteins from the “neutrophil degranulation” pathway. Instead, patients with COPD had an elevated expression of several peptidase inhibitors, higher abundance of common commensal taxa, and greater microbiome diversity.12 Although patients with COPD-BE association were most likely to have these more infected and inflammatory endotypes, compared to BE or COPD alone, disease labels did not perfectly classify patients.
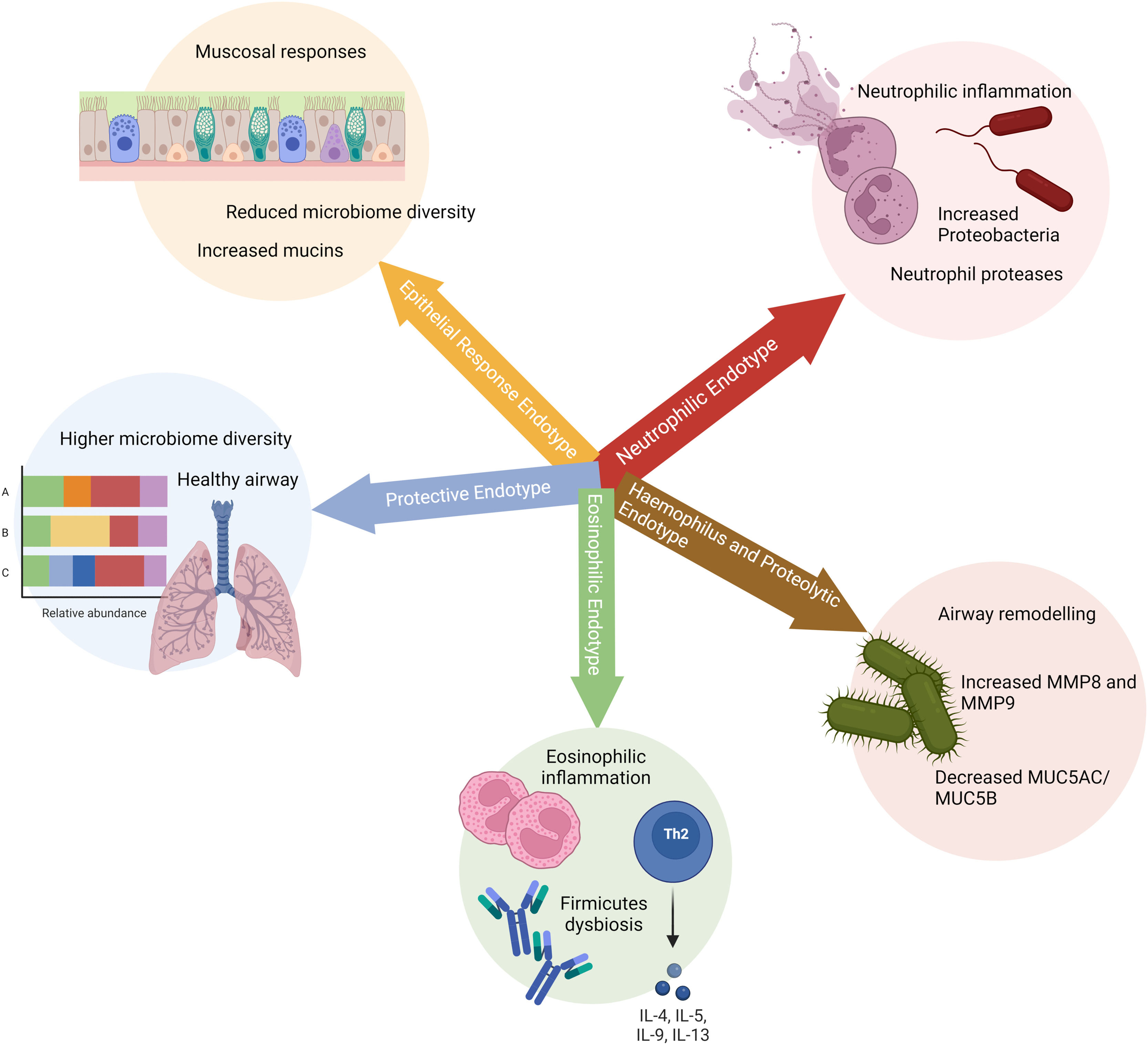
Five endotypes have been proposed with differential inflammatory, mucin, and microbiological features (Fig. 1). This information may be used for biological classification of COPD–BE association endotypes (III to V) and have potential therapeutic implications.12
Disclosed endotypes for patients with chronic obstructive pulmonary disease (COPD), bronchiectasis and COPD-bronchiectasis association. The five groups are based on: 1) neutrophilic inflammation associated to NETosis; 2) proteolytic remodelling; 3) Th2 high responses associated to eosinophilic inflammation; 4) regulated inflammation; 5) mucus hypersecretion.
Asthma is one of the most frequent BE-associated comorbidities, and this association may increase airways inflammation and exacerbation rates.13
According to Sheng et al., the coexistence of bronchiectasis predicts more severe disease in terms of asthma and chronic rhinosinusitis (CRS) and a higher incidence rate of nasal polyps.14 Furthermore, in patients with severe asthma, bronchiectasis is associated with longer asthma history and chronic airflow obstruction.15
These findings are still insufficient to consider features of asthma-BE endotypes but could possibly contribute to early recognition and targeted treatment of this patient group. Underlying asthma as an endotype is limited by two important considerations. First, asthma itself is a heterogeneous disease with multiple endotypes and is increasingly categorised into Th2 high and Th2 low subgroups, with further subdivisions based for example on the presence of specific allergy and eosinophilia. Second, the diagnosis of asthma is challenging and is sometimes inaccurate. Consequently, while it is possible to say that asthma and bronchiectasis frequently co-exist there are limited data on how this translates into underlying biology.
Gastro-oesophageal reflux diseaseGastro-oesophageal reflux disease (GORD) is a common comorbidity in bronchiectasis with a prevalence ranging from 26% to 75%. Two mechanisms are thought to contribute to bronchiectasis pathogenesis in GORD context: vagally mediated reflex bronchoconstriction and pulmonary microaspiration.16 The pathogenic role of Helicobacter pylori infection in bronchiectasis is still not fully understood, but once the microbiome in bronchiectasis is frequently dominated by enteric Gram-negative organisms, it is plausible to there be a link between the gut, the upper airway and the lung.17 Patients with co-existing bronchiectasis and GORD have increased disease severity and mortality, more frequent exacerbations, greater radiological extent, with reduced pulmonary function and quality of life.16
Identifying GORD in bronchiectasis patients may have important therapeutic and prognostic implications, although clinical trial evidence that treatment targeted at GORD can improve outcomes in bronchiectasis is currently lacking.
Inflammatory bowel diseasesPulmonary manifestations of inflammatory bowel disease (IBD) like bronchiectasis are increasingly recognized in patients with ulcerative colitis and Crohn's disease.
Although the pathogenic mechanisms are still poorly understood, evolving data suggest that there is a “lung-gut axis” and a shared antigen hypothesis behind these shared disease states. The lungs and the intestines are derived from the same embryonic cell line, the foregut region of the endoderm. Since they share a common epithelium, they may develop similar inflammatory reactions.18 On the other hand, the shared antigen theory, notes that gut and lung epithelia are exposed to the same antigens and this shared exposure may induce similar lymphoid inflammation in both systems.19
Of patients with large airways disease, about two-thirds will have bronchiectasis, which are more frequently associated to ulcerative colitis and female gender.20 As both diseases are based on lymphoid inflammation, management typically includes corticosteroid therapy, making IBD associated disease a relevant endotype with a specific treatment.21
Immunodeficiency syndromesImmunodeficiency syndromes, both primary and secondary forms, are an important and underdiagnosed cause of bronchiectasis. Primary immune deficiencies, in particular, are increasingly identified and defined as contributors, most commonly common variable immunodeficiency (CVID).22
According to Patrawala et al., more than half of the CVID patients were reported to have airway disease, including bronchiectasis.23 This is associated to low CD4+ levels, late age diagnosis and severe disease measured by pathogenic organisms such as P. aeruginosa and NTM.22,24,25
Hyper-IgE syndromes (HIESs) require gene sequencing for diagnosis once they are associated to specific gene mutations and they associate to recurrent pyogenic pneumonias during childhood culminate in structural lung abnormalities, namely bronchiectasis.26 In other immune deficiency such as X-linked agammaglobulinemia (XLA), once bronchiectasis has developed, it progresses despite IgG replacement therapy.27
Secondary forms of immunodeficiency result in bronchiectasis through a complex network of autoinflammatory and autoimmunity mechanisms.
Undoubtedly, all patients with idiopathic bronchiectasis should be screened for possible immunodeficiency. Its identification impacts therapeutic management and provides an opportunity to improve clinical outcomes.
Connective tissue diseases and other systemic autoimmune diseasesBronchiectasis is a common extra-articular feature in rheumatoid arthritis (RA), with a prevalence of approximately 20%, and it may precede articular manifestations but it is most often seen as a delayed complication of RA.28
Several hypotheses have been exposed to explain the pathogenesis of RA-bronchiectasis, including a link between autoimmunity in RA leading to airway damage, as well as recurrent infections leading to bronchiectasis.29 The later seems less likely the main mechanism as patients with other connective tissue diseases such as systemic sclerosis only very rarely develop free standing bronchiectasis.30 Risk factors for RA-bronchiectasis include older age, longer RA duration, and genetics.28 Current literature also suggests that anti-CCP antibodies (ACPA) levels are higher in patients with RA-bronchiectasis are associate with more severe lung disease.30
The frequency of bronchiectasis in Sjögren syndrome, as assessed by HRCT, varies from 7% to 54%.31 Sjögren's syndrome patients with bronchiectasis are older at the time of diagnosis, are more likely to have hiatal hernia, have a higher frequency of anti-smooth muscle antibody and a lower frequency of anti-SSA antibody than those without bronchiectasis.32
More research is needed to identify proper biomarkers to personalize treatment in systemic autoimmune diseases.
Primary ciliary dyskinesiaPrimary ciliary dyskinesia (PCD) is a genetically and clinically heterogeneous disease, and an underdiagnosed cause of bronchiectasis.33 This syndrome is caused by genetic mutations, usually inherited in an autosomal recessive pattern, affecting motile cilia, causing disease of the upper and lower airways.34
Recent advances in genomics allowed the discovery of new primary ciliary dyskinesia genes over the past decade, and >50 genes are now reported to cause PCD.35
Mutations in the gene DNAH5 result in the most frequent defect reported in individuals with primary ciliary dyskinesia.34 Genetic defects evolving MNS1, ENKUR, CFAP53 and DNAH9 genes have been recognised in individuals with motile ciliopathies that result in randomisation of left–right body asymmetry and male infertility or both, but presenting subtle or no respiratory disease.35 Reduced generation of multiple motile cilia occurs in patients with mutations in MCIDAS and CCNO resulting in a severe respiratory phenotype.36,37 DNAH11 mutations cause a shared abnormality in ciliary ultrastructure previously undetectable by transmission electron microscopy (TEM).38
As a result of the heterogeneity of PCD genetically, patients may present with classical PCD in childhood with laterality defects, whereas other patients may be missed even in adulthood because of atypical presentation and a lack of awareness.
As multidisciplinary and holistic approach to diagnostic testing is required, remarkable progress in genomics is improving diagnostic capabilities and has the potential to lead to new personalised therapeutic options.
Alpha-1 antitrypsin deficiencyAlpha-1-antitrypsin deficiency (AATd) is a hereditary disease, mainly characterized by early onset and lower lobes’ predominant panlobular emphysema. Bronchiectasis is also frequently observed in patients with AATd.39
Augmentation therapy is licensed in several countries, particularly in cases of severe disease with airway obstruction. However, there is not a clear recommendation to screen for bronchiectasis in AATd.40 The European Respiratory Society guidelines for the management of adult bronchiectasis suggest that only the presence of lower lobes emphysema or early onset airways’ obstruction could represent an indication to screen for AATd.1
Routine screening for AATd shows variable detection rates according to geographical location perhaps reflecting variable prevalence of AATd across Europe.39,41 Protease-antiprotease balance is important in the pathophysiology of bronchiectasis and with antiprotease therapies now in development it is likely it will become an even more important topic in future. Further studies are required in different geographical regions, which may have a higher prevalence of AATd, allowing personalised therapy that may improve the management in targeted patients.
There are many other underlying causes of bronchiectasis some of which have very important clinical implications. Next, we will discuss topics on airway inflammation and infection. Tuberculosis is an important cause of bronchiectasis globally but data are limited on whether this has a specific clinical presentation or endotype.
Infective endotypesMicrobiome
Understanding the contribution of airway infection to the pathogenesis of bronchiectasis is pivotal. The Cole's “vicious cycle” has been recently replaced by the “vortex cycle” model, which suggests a similar group of factors and consequences, but no sequential manner to their incitement or perpetuation in developing bronchiectasis.42 Reflecting the increasingly complexity of our understanding of bronchiectasis pathophysiology, the idea that patients are chronically infected with pathogenic bacteria has been replaced with an understanding that microbial dysbiosis, in which loss of microbial diversity and dominance of the microbiome with specific organisms contributes to disease progression. Woo et al. suggests that the lung microbiome in bronchiectasis is relatively stable over time, being highly individualized, and that lung microbial diversity may be an important contributor to clinical course.43
Present knowledge on lung microbiome in bronchiectasis is growing due to the use of next-generation sequencing (NGS) techniques in lung samples, with 16S rRNA amplicon sequencing the most commonly used technique in bronchiectasis.44 Changes in the bacteriome are associated with raised inflammatory parameters in sputum and impaired lung function.45 Studies of the sputum microbiome uncover a complex milieu of organisms including bacteria, viruses, and fungi that potentially interact within the bronchiectasis airway, which explains the inherent heterogeneity and contrasting clinical course observed between patients.46
Total airway bacterial load can classify patients depending on their response to antibiotic treatment, with high bacterial load being associated with greater lung inflammation and a greater response to inhaled antibiotics.47 Furthermore, recurrent antimicrobial use might influence microbial homoeostasis, not only disrupting the microbes and current microbial interaction networks but also increasing the emergence of microbial resistance leading into a complex and dynamic microbiological paradigm.48
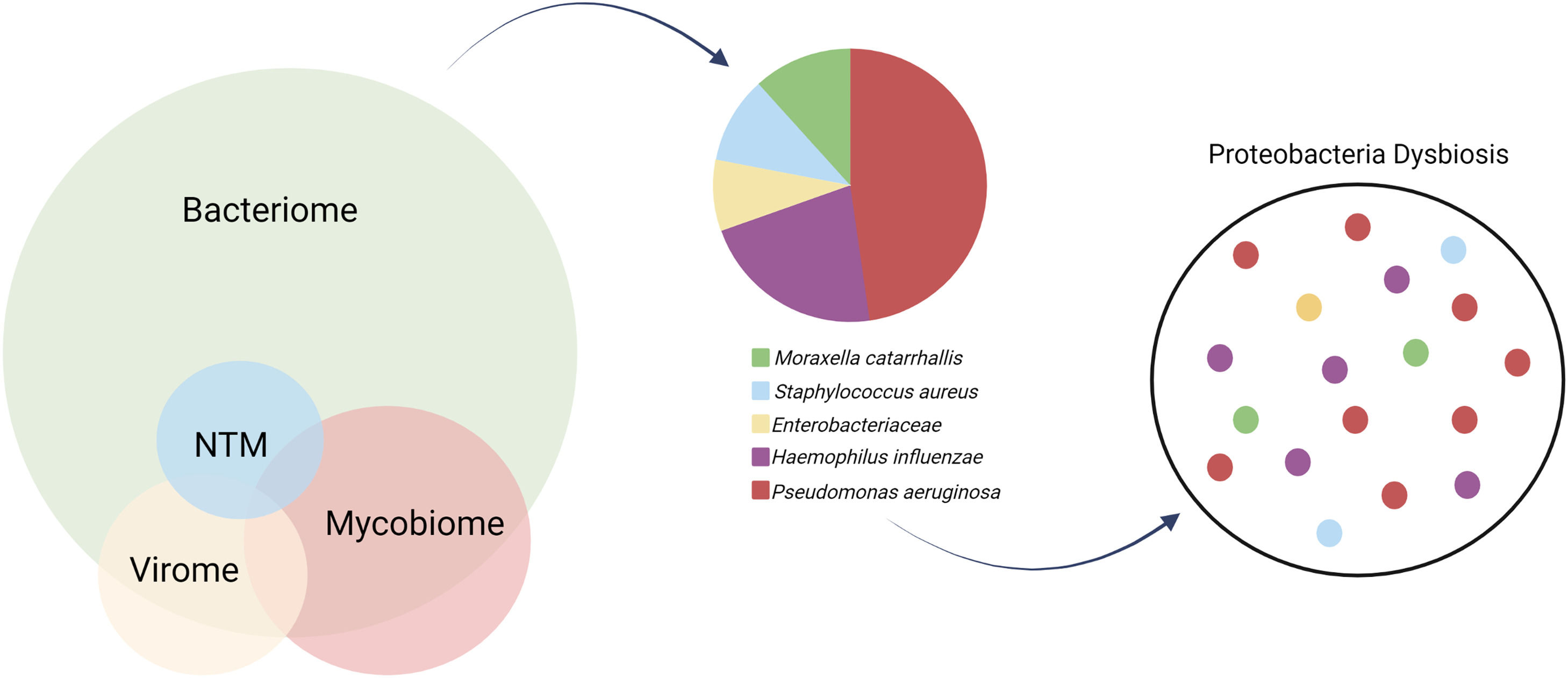
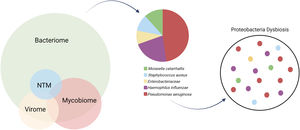
The characterization of the microbiome is vital for disentangling a clinically heterogenous endotype that converges in a mutual structural airway damage.49 It incorporates the bacteriome, virome, mycobiome and also lesser common pathogens as non-tuberculous mycobacteria (NTM).
BacteriomeThe dominant genera in a healthy airway include Prevotella, Veillonella, Fusobacterium, Streptococcus, Porphyromonas and Neisseria, all thought to be seeded into the lower airway through microaspiration from the upper respiratory tract. However, the most common organisms that chronically infect the airways of bronchiectasis patients are the Gram-negative pathogens from the Proteobacteria phylum such as Pseudomonas aeruginosa, Haemophilus influenzae, Moraxella catarrhalis, and Enterobacteriaceae, or pathogens from the Firmicutes phylum such as Staphylococcus aureus and Streptococcus pneumoniae.46 Proteobacteria dysbiosis of the microbiome, defined as dominance of these taxa, most commonly Pseudomonas and Haemophilus, is associated with more severe disease and worse clinical outcomes, indicating microbial targets for interventions.50 (Fig. 2).
The microbiome in bronchiectasis in composed by the bacteriome, mycobiome and virome. Respectably to the bacteriome, culture-based microbiology results from European cohorts show predominance for Haemophilus influenzae and Pseudomonas aeruginosa. Multiple factors might lead to Proteobacteria dysbiosis and loss of diversity in the bronchiectasis lung microbiota.
An antagonist relationship has been observed, in both culture-based and culture-independent studies, between H. influenzae and P. aeruginosa.43. These are also the two most dominant taxa identified by 16S rRNA sequencing and isolation of mucoid P. aeruginosa or H. influenzae have been shown to have the greatest influence on community structure as a whole.51 In fact, patients with P. aeruginosa– and H. influenzae– dominated communities had significantly higher serum levels of C-reactive protein (CRP), and higher sputum levels of interleukin (IL)−1β and IL-8.52
Pseudomonas aeruginosaP. aeruginosa is the most commonly identified pathogen in bronchiectasis patients worldwide. It has been associated with increased exacerbation frequency, increased hospital admission risk and worse quality of life.53 It is associated to a nearly threefold increased risk of death, with the risk strongly associated with exacerbations.54
Higher levels of active neutrophil elastase are associated to low microbiome diversity and specifically to P. aeruginosa infection.55 The induction of neutrophil extracellular traps (NETs) formation gives P. aeruginosa a survival advantage: NETs inhibit and kill competitor microorganisms, and P. aeruginosa persists by degrading NETs and evading killing by inflammatory cells.56,57 Moreover, P. aeruginosa relies on the quorum sensing (QS) signalling system as a central regulator mechanism of virulence expression that contributes to the formation and maintenance of biofilms and tolerance to conventional antimicrobials. Therefore, the persistence of this pathogen is associated with biofilm formation, innate antimicrobial resistance and resistance to host cell clearance. Consequently, P. aeruginosa infected patients are considered a distinct and stable phenotype with poor outcomes.58
Other bacteriaH. influenzae is a common but less well studied pathogen in bronchiectasis.44 It has been associated with a loss of microbial diversity and the formation of NETs as well as specific increases in matrix metalloproteinases MMP2 and MMP8.56,59
The relationship between S. aureus and non-CF bronchiectasis is yet not well established.60 Metersky et al. reported a frequency of infection similar to prior studies and that S. aureus does not appear to be an independent risk factor for severe disease in patients with bronchiectasis.61
As for S. aureus, scarce information is available about Stenotrophomonas maltophilia in patients with bronchiectasis. Metersky et al. also concluded that bronchiectasis patients with S. maltophilia may have worse outcomes than patients without this organism or without P. aeruginosa.62
ViromeFew actual studies exist that can illustrate the true extent of the virome in the setting of bronchiectasis. Respiratory viruses are commonly detected in patients with stable bronchiectasis, with elevated burdens over winter comparing to summer season.63 Respiratory viruses play crucial roles in triggering bronchiectasis exacerbations, particularly coronavirus, rhinovirus and influenza A and B.64 Despite high viral burden in stable-state bronchiectasis, it was not detected significant association between common respiratory viruses and clinical outcomes.
Whether some patients with bronchiectasis who experience frequent exacerbations have increased susceptibility to viral infection or exacerbation upon viral infection, as has been demonstrated in asthma, is unknown.
MycobiomeFungi might have a pathogenic role in bronchiectasis related to immune dysregulation and a sharp allergic response following exposure. The major clinical manifestations of fungal disease in bronchiectasis is ABPA, which affects up to 10% of the patients, being dominated by a Th2-driven response with elevated levels of total and specific IgE and eosinophilic inflammation.65
Máiz et al. showed high rates of fungal isolation and persistence in respiratory secretions of bronchiectasis patients.66 In a study using molecular methods to profile the “mycobiome”, it was determined that, while the Aspergilli remain the best characterized fungi in the bronchiectasis airway, Candida species are the most widely detected in bronchiectasis patients.67 Additionally, Poh et al. described that systemic chitinase activity, an important innate immune defence mechanism against infection, may represent a useful clinical tool for the identification of fungal-driven “frequent exacerbators” with bronchiectasis in South-East Asian populations.68
More recently, the Cohort of Matched Asian and European Bronchiectasis (CAMEB) study was the first report on the pulmonary mycobiome in bronchiectasis across continents and in age and sex-matched populations from distinct geographical regions. It provided key insights into Aspergillus-associated disease in bronchiectasis since it identifies distinct dominant mycobiome profiles according to a geographic region.69 Moreover, compared with those Aspergillus colonized and/or sensitized, patients with serological ABPA (sABPA) had more severe disease, greater exacerbations, and poorer lung function.2,69
To date, it is clear that sensitization to fungi leads to a clinically relevant and treatable endotype of disease. From a mycobiome standpoint, research suggests there are detectable fungi within the airway but their clinical correlates and usefulness in patient stratification are not yet clear.
Non-tuberculous mycobacteriaNTM can be a cause or a consequence of bronchiectasis and despite its heterogeneous prevalence due its distinct geographic distribution, it is settled that the incidence of NTM pulmonary disease (NTM-PD) is increasing worldwide.70,71
Bronchiectasis patients have a higher risk of NTM-PD compared with age- and sex-matched populations and particular phenotypic characteristics are also associated to NTM infection as pectus excavatum, scoliosis, mitral valve prolapse and lower fat mass index.72,73,74 This suggests a distinct endotype although the genetics are complex and suggest a combination of cilia and connective tissue related genes may be involved.
Patients with NTM often have coinfection with P. aeruginosa and Aspergillus-related lung disease, which suggests there may be a shared susceptibility across different infections.73
Inflammatory endotypesNeutrophilic inflammation
Neutrophils, the major cell type identified in bronchiectasis airway secretions, are mobilized to the airways via a variety of chemokines including leukotriene B4, IL-8, IL-1β and TNF-α.75 Neutrophils in bronchiectasis are dysfunctional, and predisposed to increased protease release, overwhelming anti-protease defence and failure of pathogen clearance.76
NET formation has been identified as a key mechanism of neutrophil dysfunction in bronchiectasis. NETs function is to eliminate pathogenic microorganisms, but an excess of its production leads to tissue damage and persistent airway inflammation.77
NETs release webs of neutrophil DNA into the airway with large amount of enzymes including neutrophil elastase (NE). NE is a key driver of disease in bronchiectasis with links to tissue degranulation, impaired bacterial clearance and mucus hypersecretion. Increased NE sputum levels in patients with bronchiectasis is associated with decline in FEV1, chronic infection by P. aeruginosa, high exacerbations rate and risk of mortality.56,78,79,80 As NE is one the molecules contributing to NETosis and NETs increased production is a recognised negative predictor of clinical outcomes, endotypes based on NETs or NE are promising, pointing to potential targetable therapy.81
Cathepsin C, also known as dipeptidyl peptidase-1 (DPP1), is responsible for the activation of serine proteases, like NE, in neutrophils precursors.76 Due to the fundamental role of DPP1 in serine protease activation, DPP1 inhibitors have recently been developed. Brensocatib reduced neutrophil elastase activity and prolonged the time to next exacerbation in a recent phase 2 trial.82
MMPs are proteases responsible for degrading the extracellular matrix and, so far, 28 MMPs have been described.83 Elevated sputum levels of MMP-8 and MMP-9, expressed by neutrophils, associate to more severe disease, P. aeruginosa infection as well as higher risk of future exacerbations.84
PZP is a glycoprotein and one of the molecules released by the neutrophils as part of the NET formation. Elevated sputum levels of PZP were found in exacerbator patients with severe bronchiectasis disease. Microbiome analysis revealed the predominance of pathogenic Proteobacteria, supporting that neutrophil-associated proteomic signatures predict dysbiosis.85
Overall, there are now multiple markers demonstrating the importance of neutrophilic inflammation and protease anti-protease balance in bronchiectasis. Patients with higher levels of NETs have a distinct prognosis and response to treatment suggesting a true “endotype”. Macrolides have been shown to reduce NETs and may be a marker of response.56 In addition, as NETs are strongly associated with bacterial infection, higher levels of neutrophilic inflammation may be an indication for antibiotic treatment.56
Eosinophilic inflammationTh2-driven responses have been increasingly recognized in bronchiectasis. This endotype, defined by the presence of either eosinophils blood count≥300 cells/µL or oral FeNO≥25 dpp, has been described in 20–30% of the bronchiectasis patients without asthma.86,87
Recent sputum protein profiling results disclosed that eosinophil peroxidase (EPX) is significantly elevated in severe disease and severe exacerbations. Moreover, correlation analysis confirmed EPX as an eosinophil-specific inflammatory marker.88
Blood eosinophil counts of >300 cells/μl were associated with both Streptococcus- and Pseudomonas-dominated microbiome profiles and, after controlling for infection status, Shoemark et al. showed that raised blood eosinophil counts associated with shorter time to exacerbation.87 In fact, one study suggested that 5% of non-asthmatic bronchiectasis patients with a T2-high endotype are frequent exacerbators, despite therapeutic optimization, and might be ideal candidates for anti-IL5 and anti-IL5rα treatments.86 Some observational studies have also observed the positive effect from biological therapies such as mepolizumab or benralizumab in patients with clinically relevant severe bronchiectasis and eosinophilia with both concomitant and non-concomitant asthma, with a reduction in blood eosinophils accompanied by a clinical and functional improvement in the quality of life.89,90
It is not only high levels of eosinophils which are a useful biomarker. Blood eosinophils counts of <100 cells/μl are associated with bronchiectasis severity and increased mortality, suggesting that low blood eosinophils could be a good biomarker of severity of bronchiectasis.87,91 One potential mechanism for the above is that patients with low blood eosinophils are those with more severe neutrophilic disease and this reflected in a switch in granulocyte production towards neutrophils and away from eosinophils.
The evidence accumulated on the role of bronchial and eosinophils in bronchiectasis is still very scarce, but it has already been suggested that inhaled corticosteroids (ICs) might reduce the exacerbation rate and improve quality of life in patients with bronchiectasis not related to COPD.92,93
In the context of Th2-driven responses, Mac Aogáin et al. focused their research on atopy and sensitization. The CAMEB study concluded that sensitization rates in bronchiectasis exceed those of an atopic comparator (allergic rhinitis).69 Following a comprehensive airway immune profiling, two “immunoalergotypes” were disclosed.94 One of these immunoalergotypes associates to significant worse lung function, implicating fungal exposure as a potentially treatable endotype in the implicated sub-populations.95
In summary, there is Th2-endotype of bronchiectasis which is distinguished from asthma and may be identified through blood eosinophils, raised FeNO and/or sensitization to Aspergillus or other aeroallergens. There is preliminary evidence that biomarkers such as blood eosinophils can identify responders to ICS or anti-IL5 therapies in a precision medicine approach.
Antimicrobial peptides associated inflammationAntimicrobial peptides (AMPs) are a diverse group of molecules that are important in host defence against microbes but can be proinflammatory in chronic lung disease. Sibila et al. concluded that frequent exacerbators with bronchiectasis showed dysregulated sputum AMP levels, characterised by elevated LL-37 and reduced secretory leucocyte peptidase inhibitor (SLPI). High levels of LL-37 and low levels of SLPI levels in sputum have an independent association to disease severity, P. aeruginosa infection and risk of future exacerbation.96
Recent cluster analysis allowed the identification of three endotypes based on different sputum levels of AMPs. These endotypes display distinct inflammation profiles and might hold different disease severity and risk of exacerbation.97 Still, SLPI is degraded by NE and some AMPs including LL-37 are released from neutrophils during NETosis so whether this represents distinct endotypes or alternative biomarkers of the severe NET associated endotype has not been fully established.
Systemic inflammationThe inflammatory response present in bronchiectasis is predominantly located at the pulmonary level. Nevertheless, different studies report elevated levels of systemic inflammatory markers whether in clinical stability or exacerbations and that systemic inflammation itself is a feature that has been associated with a greater degree of local inflammation and severity.98,99
White blood cells, erythrocyte sedimentation rate (ESR) and serum TNF-α all have a well-established and significant association between systemic inflammation and bronchiectasis severity.99,100 The strengths of these associations are weak and do not allow decision making at an individual patient level, particularly as systemic inflammatory markers are non-specific.
Higher CRP value is associated with a greater risk of future severe exacerbations in patients with steady-state bronchiectasis and interestingly patients were more responsive to macrolides in bronchiectasis if they had higher levels of CRP at baseline.101,102
Platelets represent a cheap and easy-to-evaluate biomarker. In stable state bronchiectasis, thrombocytosis is associated with disease severity, hospital admissions, poor quality of life, and mortality.103 Soluble P-selectin (sP-selectin), which is released from platelet membrane, plays an essential role in platelet activation. sP-selectin levels were higher in exacerbated patients compared to those in the stable state, and also higher in stable state patients compared to controls, suggesting a baseline increased platelet activation in bronchiectasis patients.104
Patients with bronchiectasis have an increased risk of mortality, particularly mortality related to cardiovascular disease.105 The associated cardiovascular risk further increases around the time of exacerbation.106 Endothelial progenitor cells (EPCs) are inversely correlated with cardiovascular risk factors and deficiencies in their number and function are present in patients with bronchiectasis, which relate to disease severity.107 Additionally, serum desmosine (sDES), which represents systemic elastic degradation and vascular ageing, is a particular predictor of cardiovascular mortality in bronchiectasis since elastin degradation may be plausible link between airway inflammation and cardiovascular risk.108
According to gender, Wang et al. reported that female patients had lower levels of inflammatory parameters except for ESR (normal ranges in both genders but slightly greater in female).109 Rather than the evaluation of a single biomarker, these authors also believe that clustering analysis of systemic parameters offers a powerful tool to better characterize patients with bronchiectasis. Using a data mining approach, they were able to define three clusters which significantly correlated to disease severity, indicating that these results have clinical implications in the management of the complexity and heterogeneity of bronchiectasis patients.110
Mucus, mucins and mucociliary dysfunctionMucus is a protective coating secreted in the healthy airways, composed of water, salt and proteins. Mucins are glycoproteins responsible for the protective and clearance properties of the mucus. MUC5AC and MUC5B are most abundant and important airway mucins.111. Mucin concentration is significantly higher in patients with bronchiectasis than healthy individuals and it related to osmotic pressure, greater viscosity and inflammation, and infection.112
Mucus and cilia form the mucociliary escalator, ensuring that airway's foreign agents are transported and either swallowed or expelled by coughing.113 Factors that may cause reduced ciliary beating include cyanide produced by P. aeruginosa and neutrophil proteases.114 Recently, it has been proposed that impairment of mucociliary clearance might result from both Th1 (IL-1β, IL-8) and Th2 (IL-4 and IL-5) inflammatory cytokines.115 MUC5AC release is also closely related to airway inflammation. Therefore, while impaired mucociliary clearance may contribute to some endotypes, they are closely linked to the inflammatory endotypes described above with both NET associated and Th2 high endotypes causing ciliary dysfunction and mucus hypersecretion.
GeneticsIt is likely in the coming years that whole genome sequencing will identify new genetic causes of bronchiectasis. Although most patients will be diagnosed with idiopathic bronchiectasis, reviewing conditions with well-known genetics-based pathways offers insights into understanding the underlying mechanisms of bronchiectasis pathogenesis.42
Mannose-binding lectin (MBL) is a key component of innate immunity involved in clearance of bacteria and apoptotic cells. Genetic MBL deficiency is common in the general population and related to disease severity in bronchiectasis, including quality of life and frequency of exacerbations and admission to hospital.116
Secretion of α(1,2)fucosylated glycans elicits a dichotomous effect on host–microbe interactions, making the secretor genotype (FUT2) a risk factor underlying variation in infection type and disease severity in bronchiectasis. Homozygous secretors exhibit lower lung function, higher exacerbation rate and more frequent P. aeruginosa-dominated infection.117
Telomere attrition is an established ageing biomarker. Lim et al. reported that shortened telomere length was significantly relevant in sputum immune cells of bronchiectasis patients and that gene GBP5 upregulation, a positive regulator of the NLRP3 inflammasome, led to exaggerated immune response upon bacterial infections.118
Most studies of genetics in bronchiectasis to date are limited to a few hundred patients whereas breakthroughs in the genetics of other complex diseases have required studies of several thousand patients. As large cohorts are increasingly established with associated biobanks it should be possible to gain a deeper understanding of the contribution of genetics to endotype in bronchiectasis.
ConclusionThe heterogenous nature of the bronchiectasis in underlined by multiple endotypes which may represent different treatable traits. It is essential to define the biological pathways leading to airway inflammation and disease progression, by using the available “omics” technologies, so that novel biomarkers are identified and personalized therapies are developed.
Future results from international multi-omics studies such as the Bronchiectasis Research Involving Databases, Genomics and Endotyping (BRIDGE) study (ClinicalTrials.gov Identifier: NCT03791086), promoted by EMBARC, will allow us to identify and characterize subpopulations of patients with bronchiectasis, through stable state or exacerbation, in order to obtain meaningful outcomes.