In late December 2019, clusters of patients with interstitial pneumonia of unknown cause were reported by some local health facilities in Wuhan (China). The Chinese Centre for Disease Control conducted an epidemiologic and etiologic investigation, leading to the identification of a novel coronavirus (SARS-CoV-2).1,2 On March 11th, the World Health Organization (WHO) declared the novel coronavirus disease (COVID-19) a pandemic. In the area of Wuhan, COVID-19 mainly affected male patients (around 60%), with a median age of about 50 years; 40% of patients developed Acute Respiratory Distress Syndrome (ARDS) 5% requiring intensive care. The mortality rate was around 2%.3,4 However, Grasselli et al. found that the mortality was 26% in ICU. The death rate was higher among those who were older.5
In a more recent report from Italy including 22512 patients, COVID-19 has infected 2026 healthcare workers, with a total case fatality rate of 7.2%. Patients were predominantly older than 60 years, 46.1% had mild severity, while 24.9% severe disease.6
To date (April 16th) the cases are 1991562 with more than 130000 deaths.7 In a certain percentage of patients, COVID-19 is a viral interstitial pneumonia8 characterized by fever, dry cough, dyspnoea, and bilateral ground-glass opacities,9 with about 67% of patients evolving to a severe pneumonia.10,11
However, preliminary observations reported that COVID-19 patients, compared to conventional ARDS, are characterized by moderate to severe hypoxaemia despite a relatively high pulmonary compliance.12,13 A potential mechanism may be loss of hypoxic vasoconstriction, explaining the observed severe hypoxaemia and the effect of very high levels of positive end-expiratory pressure (PEEP) on oxygenation not depending on lung recruitment.13 High levels of PEEP may adjust redistribution of perfusion diverting flow towards high ventilation-perfusion (Va/Q) areas increasing arterial oxygen tension (PaO2); however, over-distension of the healthy lung areas and an increased right cardiac afterload is possible.13
In patients with mild to moderate ARDS, with a PaO2 to inspired oxygen fraction (PaO2/FiO2) >150, different modalities of non-invasive respiratory support (NIRS) might be attempted in order to avoid intubation.14,15 However, NIRS could potentially lead to intubation delay and cause a self-inflicted lung-injury (SILI),16 due to the high transpulmonary pressures. SILI in turn would lead to a severe decrease in lung compliance.17 Continuous Positive Airway Pressure (CPAP) is a form of NIRS during which a fixed level of PEEP is applied to the airways, while the entire work of breathing is generated by the patient's respiratory muscles (i.e. no pressure assist is provided during inspiration). This would reduce the likelihood of generating high transpulmonary pressure and tidal volume compared to non-invasive intermittent positive pressure ventilation.18
Due to the enormous number of COVID-19 patients with acute respiratory failure and to the shortage of ICU beds and ventilators, helmet CPAP (hCPAP) is widely used in Italy.5,19
In particular, in a scenario of a discrepancy between facilities and a large number of casualties, as with COVID-19 pandemic, the application of hCPAP might be useful as an “easy to perform” supportive strategy.
Prone position sessions may adjust pulmonary perfusion diverting flow towards high Va/Q areas, and allowing a redistribution of aerated and non-aerated areas whenever present.20,21 Furthermore, as opposed to non-invasive intermittent positive pressure, hCPAP does not necessarily need a ventilator (potentially in short supply in case of mass casualties) and it is not affected by patient-ventilator asynchrony, a determinant of discomfort and treatment failure.22–24
The hypothesis is that in case of a pandemic, selected COVID-19 patients may benefit from the combination of early hCPAP and prone position sessions, in order to reduce the need for intubation and invasive mechanical ventilation, “buying time” for the disease to heal.
Evaluation of the hypothesisProne positionProne position was first described in 1976 in patients with ARDS.25 First, prone position modifies respiratory mechanics. In particular, the ventral chest wall cannot expand, because it is in contact with the firm surface of the bed.26 In patients with ARDS, the lung weight increases by 4–5 times, pulmonary tissue becomes stiffer and compliance decreases, in association with compression atelectasis.26,27 During prone position, decreased chest wall compliance improves the redistribution of lung density from dorsal to ventral areas, and increases lung aeration from ventral to atelectatic dorsal regions, improving gas exchange.28 Nowadays, the application of prone position is recommended in most severely ill patients. Guerin et al. showed that, in patients with severe ARDS, the application of prolonged (17h) prone position sessions for approximately 4 days reduced the absolute mortality risk by 17% and the relative risk by 50%.29 However, other studies have not shown outcome benefits of prone position.30–33
These differences might be explained by the fact that patients included were not so severely ill, periods of use were shorter and the use of protective ventilation strategies were less strictly enforced.34 Early application of prone position for prolonged (up to 16h) periods has been also demonstrated to improve the survival rate29 in other clinical settings.
It has been suggested that prone position in COVID-19 patients may lead to overwork of professionals with scarce clinical efficacy in terms of recruitment.13 However, preliminary data suggest that COVID-19 patients undergoing CPAP may benefit from this treatment with even the most severe forms of hypoxaemic respiratory failure characterized by a refractory hypoxaemia (i.e. PaO2/FiO2<150).5
Our hypothesis is that, selected COVID-19 patients may benefit from the combination of early hCPAP at moderate levels of PEEP (i.e. 10 cmH2O) and prone position, to avoid overdistension of the healthy lung areas thus slowing the progression of the disease and allowing patients to “buy time” to heal. Indeed, in these instances, hCPAP is likely be effective by keeping the lung open20 and reducing venous admixture by diverting flow towards better high Va/Q areas.21
Non-invasive respiratory supportAlthough life-saving, invasive mechanical ventilation is associated with side effects and complications leading to increased morbidity and mortality. Therefore, alternative strategies have been proposed, especially for those patients with less severe forms of ARDS. Among these strategies, NIRS might play a role in reducing intubation rate.14 Application of positive pressure to the airway may open collapsed alveoli, increases functional residual capacity and improve the Va/Q match and lung compliance. As a result, oxygenation and respiratory workload improve, with the potential benefit of avoiding intubation and invasive mechanical ventilation.14 More recently, the combination of non-invasive intermittent positive pressure with prone position was shown to prevent the need for intubation in up to half of the patients with moderate to severe ARDS. In addition, patients failing NIRS and requiring intubation were more severe, as compared to those succeeded.35
The use of CPAP may provide the application of a stable level of positive airway pressure throughout the entire respiratory cycle. Therefore, it may result in effective recruitment of closed alveoli, with an increase in the functional residual capacity and improvement of oxygenation.36,37 During NIRS, comfort is one of the determinants of treatment success or failure.38 CPAP may be delivered through different interfaces, such as masks or helmets. Compared to masks, helmets are more comfortable,39 they allow longer continuous application of the treatment and lower complications correlated to the interface (i.e. eye irritation, gastric distension and skin necrosis).39 As during NIRS, comfort is one of the determinants of treatment success or failure38,39 it is important to note that unintentional leaks are kept to a minimum during hCPAP.40–42
The “helmet bundle” in COVID-19 patients has recently been published to optimize treatment.19
Precautions when using NIRSIt is worth noting that the recent guidelines on the use of NIRS in de novo hypoxaemic acute respiratory failure do not provide any recommendation, due to uncertain and conflicting evidence.43 Very recently, the Surviving Sepsis Campaign has provided some guidelines on the management of critically ill COVID-19 patients.44 The panel of experts suggested a trial of NIRS, recommending close short-interval monitoring for worsening of respiratory status and early intubation in a controlled setting if worsening occurs,44 although the main risk of using NIRS in de novo ARF is delay in intubation44 with the risk of developing SILI.16,17
NIRS in the era of COVID-19Non-invasive intermittent positive pressure requires the use of mechanical ventilators, of there is currently a shortage due to the pandemic COVID-19.3,6 Furthermore, non-invasive intermittent positive pressure may also worsen patient-ventilator interaction and synchrony, which might be detrimental for patients’ comfort, leading to treatment failure.22–24 For these reasons, CPAP might be a valid alternative. In addition, the use of an interface such as the helmet may be advantageous, compared to a facial mask.45–47 In fact, the helmet improves comfort of the patient, assures prolonged continuous application of the treatment and it is characterized by very low air-leaks,40,45–48 limiting the spread of the virus in the environment. Interestingly, when a patient coughs, he/she generates a peak cough flow up to 400 L/min, theoretically creating less contamination for the operators and environment. High flow nasal therapy could be also combined with hCPAP.49,50 However, experimental studies of exhaled air dispersion by mannequins have demonstrated greater exhaled air dispersion with conventional low flow nasal cannula at 5l/min, compared with HFNC.51 Furthermore, the helmet's ports can be protected with two antimicrobial filters, further reducing air dispersion. This is of utmost importance in cases of infections transmitted by aerosolization, such as COVID-19.41
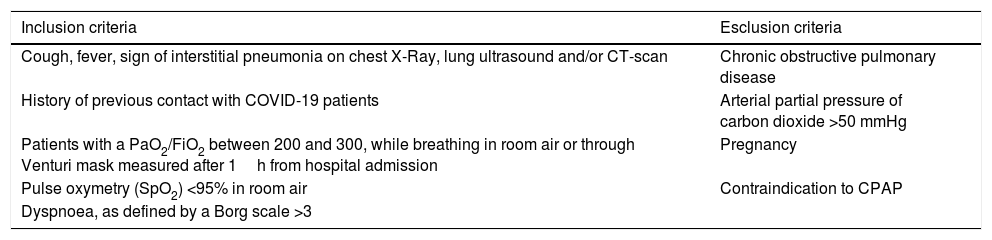
Based on these facts, we have hypothesized that, in the current COVID-19 pandemic emergency, an attempt to combine early hCPAP and prone positioning sessions might improve oxygenation in selected patients. Criteria to attempt hCPAP (8–12cmH2O) and prone position in fully collaborating symptomatic patients are listed in Table 1. The presence of dyspnoea, as defined by a Borg scale >3,52 is not deemed necessary because dyspnoea is not always clinically evident in these patients. Patients with chronic obstructive pulmonary disease or with an arterial partial pressure of carbon dioxide >50mmHg will be excluded.
Inclusion and exclusion criteria.
| Inclusion criteria | Esclusion criteria |
|---|---|
| Cough, fever, sign of interstitial pneumonia on chest X-Ray, lung ultrasound and/or CT-scan | Chronic obstructive pulmonary disease |
| History of previous contact with COVID-19 patients | Arterial partial pressure of carbon dioxide >50 mmHg |
| Patients with a PaO2/FiO2 between 200 and 300, while breathing in room air or through Venturi mask measured after 1h from hospital admission | Pregnancy |
| Pulse oxymetry (SpO2) <95% in room air | Contraindication to CPAP |
| Dyspnoea, as defined by a Borg scale >3 |
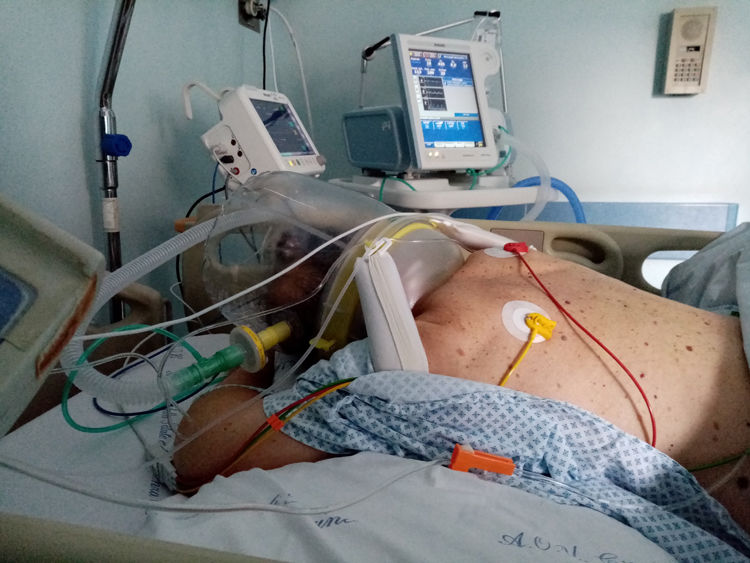
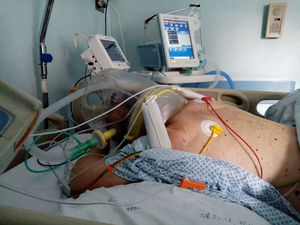
Prone position during hCPAP requires some precautions, in order to avoid discomfort, skin and eyes lesion and treatment failure. First, the use of helmet without armpit braces is preferable, although not mandatory, since in the literature it has been reported to be more comfortable.48,53 Another important precaution is to prevent the rigid collar from generating skin lesions by direct pressure on skin due to ischaemia or shearing forces and mechanical stress to the neck. Interestingly awake patients during hCPAP may assume prone position with minimal assistance. Fig. 1 shows one patient switched from supine to prone position using hCPAP and continuous tidal volume measurement using a dedicated software built into a turbine driven ventilator.54
Consequence of the hypothesis and discussionThis is the first proposal of a study aimed at investigating the possibility of combining hCPAP and prone position in order to avoid deterioration of gas exchange and intubation in patients affected by COVID-19 pneumonia.
The application of early sessions of pronation in patients with mild-to-moderate ARDS might improve gas exchange without further increasing PEEP.26,27 This experimental plan has several strengths. First, a thorough literature review and straight-forward protocol definition will guarantee the best possibilities for the intervention. The experimental treatment has notable possibilities of being effective and helpful, in particular in a setting of mass-casualties happening now in Italy and rest of Europe. Second, the ability to limit the treatment to selected patients may amplify the potential benefits reducing the failure rate. Third, if the combination of hCPAP and prone position reduced the intubation rate, the health care system could improve the allocation of ICU beds, granting better treatment to all patients needing ventilatory assistance.
Furthermore, our preliminary results (Gregoretti et al., unpublished data) from an ongoing pilot study in COVID-19 patients, measuring tidal volume during hCPAP,54 showed that a low mean tidal volume coupled with high pulmonary compliance and a low respiratory rate, which suggests that transpulmonary pressure is kept low.
Our hypothesis has also some major limitations. First of all, the real effect of hCPAP from the pathophysiological point of view in this disease is unknown. In healthy patients, CPAP in prone position causes a Va/Q mismatch for a more uniform ventilation distribution, despite a higher perfusion in dependent parts of the lung.33,55 CPAP may increase alveolar pressures and resistance in small vessels of the lungs (zone 1)56 especially in nondependent lung regions, explaining the higher perfusion in dependent parts when the subject is prone. This finding suggests the redistribution of perfusion could improve oxygenation in patients lacking hypoxic vasoconstriction. Second, inclusion criteria for this treatment are untested; in addition, time in prone position from our preliminary data is shorter than in sedated patients. Third, strict monitoring by trained personnel, in a step-down unit or in a monitored unit, would be required to early identify a treatment failure and avoid any intubation delay. Lastly, patient's tolerance may play a fundamental role in the treatment. In addition, the lack of patient-ventilator asynchronies during CPAP together with the use of the “helmet bundle” should ameliorate tolerance.41
In conclusion, if our hypothesis is valid, physicians may reduce the need for endotracheal intubation and invasive mechanical ventilation, shortening the hospital length of stay and improving survival rates. Furthermore, the need for ICU beds may be reduced, in favour of sub-intensive beds. This setting does not exclude the need of implementation in areas where prompt intubation can be easily performed. In addition, this strategy may also be an adjunctive tool for those patients who are not recommended for endotracheal intubation and care is limited to hCPAP as “ceiling of treatment”.
Otherwise, patients treated with hCPAP presenting with clinical signs of excessive inspiratory effort, should be promptly intubated to avoid too injurious transpulmonary pressure leading to SILI.16
Conflicts of interestDr. Navalesi's research laboratory has received equipment and grants from Maquet Critical Care, Draeger and Intersurgical S.p.A. He also received honoraria/speaking fees from Maquet Critical Care, Orionpharma, Philips, Resmed, MSD and Novartis. Dr. Navalesi contributed to the development of the helmet Next, whose licence for patent belongs to Intersurgical S.P.A., and receives royalties for that invention. Dr. Longhini and Dr. Navalesi contributed to the development of a new device, whose patent is in progress (European Patent application number EP20170199831). Dr. Cortegiani, Dr. Accurso, and Dr. Gregoretti declare a patent pending, in association with the University of Palermo – Italy (No. 102019000020532 – Italian Ministry of Economic Development) related to the content of this manuscript. Dr. Gregoretti received fees for lectures by Philips, and received payments by Philips for consultancies in the developing process of the EVO Ventilator and fees for lectures or consultancies from Resmed, Vivisol and Air Liquide not related to the present work. The remaining authors have no conflict of interest to disclose.
Sources of supportNone.
FundingNone declared.