Re-expansion pulmonary edema (RPE) is a rare clinical condition with a low incidence rate, which normally occurs with the rapid expansion of the collapsed lung after drainage of the pleural cavity. It often manifests with acute respiratory failure, in some cases making invasive mechanical ventilation (IMV) necessary.
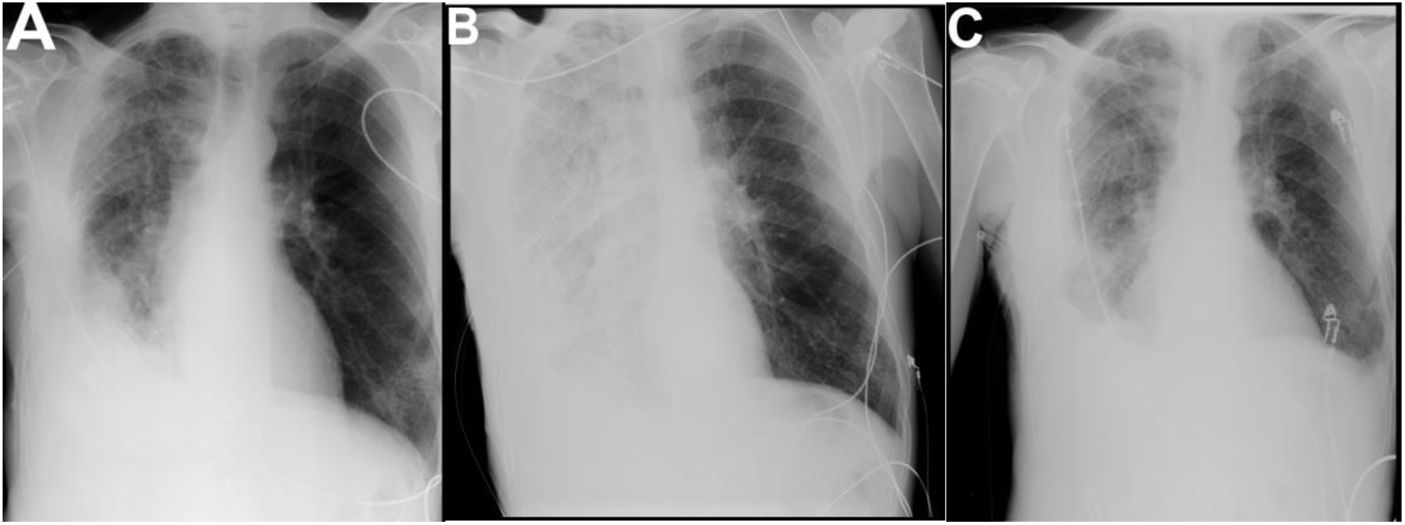
The authors report a case of a 49-year-old male, smoker of 20 packs per year, who went to the emergency department with fever, dyspnea, cough, purulent sputum, and right posterior pleuritic chest pain with 4 days of evolution. Physical examination revealed cachexia (BMI of 14.8 kg/m2), tachycardia, tachypnea, and decreased breath sounds in the lower half of the right lung field. Blood gas revealed type 1 respiratory failure (paO2/FiO2 223) and analyses identified leukocytosis with neutrophilia and increased inflammatory parameters (C-reactive protein 39 mg/dL, normal < 0.5 mg/dL). The chest radiograph revealed pulmonary homogenous opacification of the right lung, with obliteration of right costophrenic angle and superior concavity, compatible with pleural effusion (Fig. 1A). The thoracic computed tomography (CT) evidenced a large loculated right pleural effusion with pleural thickening, compatible with empyema (Fig. 1B), and extensive centrilobular and paraseptal emphysema with bullous dystrophy of apical predominance (Fig. 1C). For infection focus control, a 24 Fr chest tube was placed, with drainage of 2000 mL of purulent pleural fluid. The analysis of this liquid revealed leukocytes 28868/mm3, glucose < 5 mg/dL, LDH > 3100 IU/L, pH 6.0. The chest radiograph (Fig. 2A) after drainage, showed improvement of the pleural effusion. At the same time, empirical antibiotic therapy (ceftriaxone and clindamycin) was started.
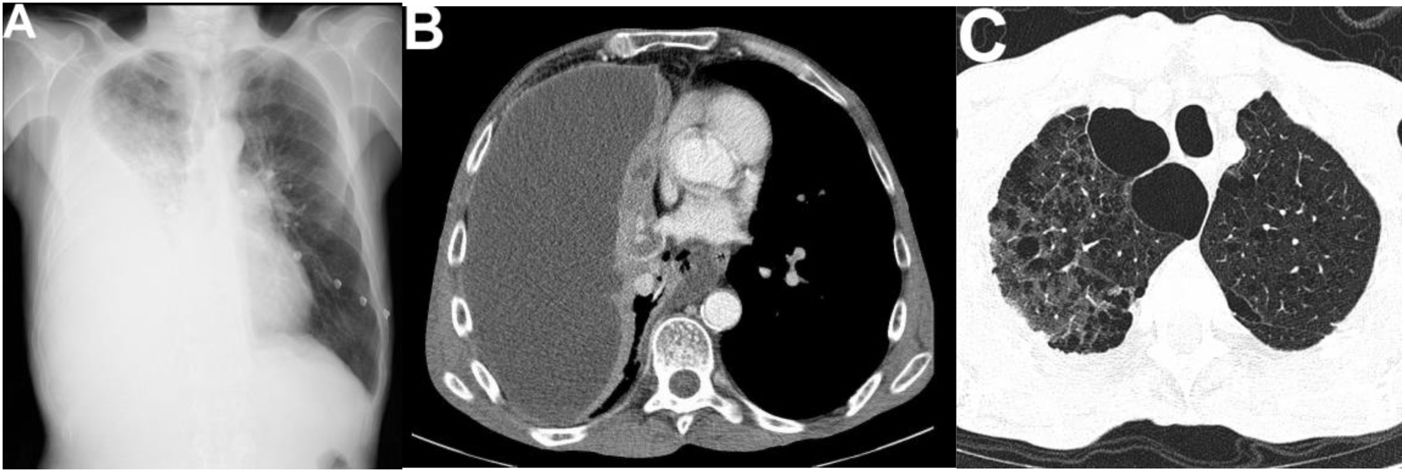
Imaging exams on admission.
A) Posteroanterior chest radiograph: pulmonary homogenous opacification of the lower two thirds of the right lung, with obliteration of right costophrenic angle and superior concavity, compatible with pleural effusion.
B) Thoracic computed tomography: large loculated right pleural effusion (200 × 74 mm in the axial plane) with pleural thickening, compatible with empyema.
C) Thoracic computed tomography: extensive centrilobular and paraseptal emphysema with bullous dystrophy of apical predominance.
One hour after the placement of the chest tube, the patient presented marked dyspnea with sudden worsening of the respiratory failure (paO2/FiO2 120). The radiograph was repeated (Fig. 2B) and revealed an opacification of the entire right lung field compatible with RPE. Due to the worsening of respiratory failure, he was admitted to the Intensive Care Unit (ICU). Because of the low BMI, the sarcopenia, and the extensive pulmonary emphysema with bullous dystrophy, IMV and non-invasive ventilation (NIV) were delayed and High-Flow Nasal Oxygen (HFNO) was initiated with the following parameters: gas flow 60 L/min; FiO2 70%. Concomitantly, diuretic therapy was started, as the administration of diuretics can diminish the occurrence of pulmonary edema by raising the osmotic pressure and decreasing pulmonary blood flow.1 The patient showed progressive recovery under HFNO over the 72 h after admission. He was discharged from the ICU on the 4th day of hospitalization, under oxygen mask (FiO2 31%) and improvement of the respiratory failure (paO2/FiO2 319). The chest radiograph (Fig. 2C) at discharge from the ICU reveals an almost complete resolution of pulmonary edema.
RPE is an uncommon condition, with an incidence rate between 0 and 1% after rapid pulmonary re-expansion of a collapsed lung.2 It presents a sudden clinical installation, which can cause severe hypoxemia and mortality rates that can reach 20%.3,4 It is usually unilateral, occurring after the active drainage of a large amount of air or liquid from the pleural cavity and risk factors for its occurrence are young age, prolonged pulmonary collapse (usually greater than 72 h), and rapid pulmonary re-expansion.4,5
The pathophysiological mechanism of RPE is multifactorial and is not fully understood. At its base is the increase in vascular permeability secondary to damage to capillaries and alveolar membrane, as well as the decrease in surfactant production, airway obstruction, changes in pulmonary artery pressure, and the production of inflammatory mediators (IL-8 and Leukotriene B4).6,7
In the case presented, the patient had decreased BMI, pulmonary emphysema, and extensive bullous dystrophy and was being a poor candidate for IMV and NIV due to the risk of barotrauma. Therefore, and taking into account the severe respiratory failure (paO2/FiO2 120), HFNO was implemented.
HFNO is a high-flow oxygen system that allows the administration of up to 60 L/min of heated and humidified gas with a variable FiO2 (21%-100%). Studies have shown that, compared to conventional oxygen therapy, HFNO allows for better oxygenation and greater comfort, which is justified by its physiological effects: generation of positive pressure at the end of expiration (PEEP), improvement of the inspired oxygen fraction, wash-out and reduction of pharyngeal dead space, reduction of respiratory work and improvement of mucociliary clearance.8
HFNO provides spontaneous breathing, maintaining positive pressure in alveoli and stable supply of a high concentration of oxygen.9 It is a simpler and more comfortable application than NIV and avoids complications associated with the latter therapy such as abdominal distention, aspiration of gastric content, facial injuries, and barotrauma.
Frat et al.10 demonstrated a decrease in mortality at 90 days (without increasing the rate of intubation) in patients with type 1 respiratory failure and paO2/FiO2 ratio < 200 treated with HNFO compared to patients treated with NIV.
In exacerbated COPD patients, HFNO has been used as an alternative to NIV. In these patients, work of breathing is reduced with HFNO by a similar extent to NIV, while keeping similar PaCO2 values. HFNO has the advantage of being more comfortable than NIV because it does not require a "true" interaction or synchrony between the system and the patient.11
In comparison with IMV, it reduces the period of treatment and hospitalization in the ICU since ventilatory weaning is not necessary, as well as reducing the incidence of ventilator-associated pneumonia and barotrauma.
In the case described, the patient's particular symptoms made him a poor candidate for mechanical ventilation, with HFNO providing the necessary respiratory support and allowing a complete recovery from the RPE episode.
In conclusion, RPE is a rare and potentially fatal clinical condition and timely diagnosis is essential to ensure proper treatment. The therapeutic is based on organ support, with a special focus on pulmonary support, and mechanical ventilation is the cornerstone of this approach. The results obtained with HFNO in this9 and other cases of type 1 respiratory failure seem promising, however, more studies are needed to establish HFNO as a safe approach to RPE.
Conflicts of interestThe authors have no conflicts of interest to declare.