Not every individual exposed to Mycobacterium tuberculosis becomes infected. One host genetic factor, involved in modulating the immune response that has been studied in many ethnic groups is the association of human leukocyte antigens (HLA) with susceptibility to tuberculosis (TB).
ObjectiveTo investigate the association between TB, HLA-DRB1 and HLA-DQB1 alleles in a Portuguese population.
MethodsHLA-DRB1 and HLA-DQB1 gene polymorphisms were analyzed by PCR-SSP in 92 TB patients, and 82 healthcare professionals without TB but exposed on a daily basis to infectious patients for more than two years (healthy exposed - HE). Tuberculin skin test reaction (TST), was positive in 69 individuals (all over 15mm) in the HE group (HE+) and negative in thirteen (HE−).
ResultsHLA-DRB1*14 frequency is higher in the TB patients group (7 % vs. 0; p=0.038) than in HE+.
ConclusionsNo genetic marker clearly indicative of disease susceptibility or resistance was identified in this study. However, HLA-DRB1*14 was more frequent in TB patients suggesting that it may be involved in the evolution infection towards active TB in our population.
Nem todos os indivíduos expostos ao Mycobacterium tuberculosis ficam infectados. Um dos factores genéticos envolvidos na modulação da resposta imune e estudado em muitos grupos étnicos é a associação entre moléculas HLA (human leukocyte antigens) e a susceptibilidade à tuberculose (TB).
ObjectivoInvestigar a relação entre TB e os alelos HLA-DRB1, DQB1 numa população Portuguesa.
Métodos: Os polimorfismos dos genes HLA-DRB1 e HLA-DQB1 foram analisados por PCR-SSP em 92 doentes com TB e 82 profissionais de saúde saudáveis, expostos diariamente a doentes baciliferos por um período superior a 2 anos (expostos saudáveis: ES). Neste grupo de ES, o teste tuberculínico foi positivo (TST=10mm) em 69 indivíduos (todos com valor superior a 15mm) (ES+) e negativo (TST<10mm) em 13 (ES−).
ResultadosA frequência do alelo HLA-DRB1*14 é superior no grupo de doentes com tuberculose em relação ao grupo de ES+ (7 % vs. 0; p=0,038).
ConclusõesNão foi identificado neste estudo, nenhum marcador genético de susceptibilidade/resistência à doença. No entanto, o alelo HLA-DRB1*14 foi mais frequente nos doentes com tuberculose, sugerindo que possa estar envolvido na evolução da infecção para tuberculose activa na nossa população.
Infection with Mycobacterium tuberculosis (MT) results in a variety of conditions ranging from asymptomatic infection to active tuberculosis (TB) with pulmonary or extrapulmonary involvement. In extreme cases MT infection may be fatal. One third of the World's population is infected with MT;1 however, only a minority ever develop clinical disease. In 90 % of infected individuals, bacilli remain under control in a latent state 2 (latent TB infection).
The various clinical features of TB result from cell-cell interactions that are promoted by cytokines produced by immune cells in response to MT infection. Studies of the diverse consequences of infection in twins 3 and under similar exposure conditions in a familial context 4 suggest the importance of genetics in susceptibility and/or resistance to TB. Several case-control studies have identified associations between TB disease and gene polymorphisms. Among the candidate genes potentially involved in the immune response to TB are the murine natural resistance-associated macrophage protein 1 (NRAMP1) gene, 5 the vitamin D receptor (VDR) gene, 6–8 tumor necrosis factor alpha (TNFα, 9 IL-10, 10–12 and IL-1. 13
HLA class II molecules are crucial in modulating the adaptative immune response, and their association with various diseases, including TB, has been described. However, results have been controversial concerning to TB, 14–20 with ethnic and/or geographic variations 21,22 apparently playing a major role in such discrepancies. The majority of published studies do not report MT exposure status in the control group and lack information about the role of HLA alleles and the outcome of TB infection.
In Portugal, the incidence of TB has been steadily decreasing since 1985, reaching a frequency of 25.3 per 100,000 individuals in 2008 23 still higher than in the rest of the European Union. 24
Some hospitals were once TB sanatoriums, and for several years maintained an important tradition of inpatient TB treatment. Until recently, there were no special measures to prevent against nosocomial transmission, and well-equipped isolation rooms have only become available in the last decade. However, after having worked in an inpatient setting with high TB exposure and without special conditions during the sanatorium phase, most are disease-free and/or uninfected.
The aim of this study was to evaluate allelic associations with outcome of TB exposure, particularly in healthcare professionals heavily exposed to TB in the past, focusing on the importance of HLA-DRB1 and HLA-DQB1 alleles.
MethodsSubjectsAll individuals were vaccinated with bacillus Calmette-Guerin (BCG) at birth, according to the national guidelines.
PatientsNinety-two unrelated TB patients (33 women and 59 men) at the Pneumologic Diagnostic Center of Vila Nova de Gaia (CDP), Portugal, were studied. All had newly detected, active pulmonary TB diagnosed using standard clinical, radiographic and bacteriological criteria. The diagnosis of TB was confirmed in all patients by positive sputum culture of M. tuberculosis (Table 1).
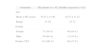
Characteristics of TB patients, healthy-exposed individuals and healthy controls
| Parameter | TB patients (n = 92) | Healthy-exposed (n = 82) |
| Age | ||
| Mean ± SD (years) | 45.47 ± 15.40 | 42.57 ± 11.42 |
| Range | 21-82 | 25-72 |
| Gender | ||
| Female | 33 (36 %) | 69 (84 %) |
| Male | 59 (64 %) | 13 (16 %) |
| Positive TST | 92 (100 %) | 69 (75 %) |
Eighty-two healthcare workers without active TB disease who had been exposed on a daily basis (for more than 8 hours a day in a confined environment) to infectious patients (inpatient and outpatient settings without protective
measures against nosocomial TB) for more than two years prior to 2000 were recruited (healthy exposed - HE). All were asymptomatic and presented normal pulmonary radiographies. Based on the results of tuberculin skin test (TST), 69 were found positive (HE+) and thirteen negative (HE—). HE+ positive results ranged from 15 to 20mm.
Inclusión and exclusión criteriaIndividuals belonging to any high-risk groups (e.g., drug users, sex workers, homeless) and persons with co-morbidities that would interfere with TST results or increased risk for TB, such as those with immunosuppressive disease (e.g., HIV infection, rheumatoid arthritis or cancer) or taking any immunosuppressive drug (e.g. corticosteroid) were excluded from the study.
Tuberculin skin testTrained nursing staff from CDC administered TSTs (2-TU dose of purified protein derivative RT23) according to the guidelines established by the Portuguese Pneumologic Society (SPP). 25 Briefly, 0.1ml of the tuberculin purified protein derivative containing five tuberculin units was injected intradermally on the volar surface of individuals’ forearms. After 72h, the diameter of the indurated area surrounding the injection site was measured and reported in millimeters. We considered 10mm as the positive cut-off value.
HLA genotypingPeripheral blood samples (10ml) were collected in EDTA tubes. Genomic DNA was obtained from Proteinase-K treated peripheral blood leukocytes using a salting-out procedure. 26
DNA was amplified by polymerase chain reaction (PCR) using sequence-specific primers (PCR-SSP) for HLA-DRB1 and HLA-DQB1 genes, based on methods previously described. 27
PCR products were electrophoresed on 1.5 % agarose gels containing ethidium bromide, and visualized under ultraviolet light.
Statistical analysisHLA-DRB1 and HLA-DQB1 phenotypic frequencies were determined by direct count. Comparisons of HLA frequencies between patients and controls were performed using the Pearson χ2 Test with continuity correction or the Fisher's Exact Test when an expected absolute cell frequency was less than 5; p values < 0.05 were considered statistically significant, with relative risk defined using a 95 % confidence interval (95 %CI). No multiple comparisons adjustments were considered in assigning values for significant differences. All analyses were done with SPSS v.16 software (Statistical Product and Serve Solutions).
ResultsHLA-DRB1 allelesIn the current study, there were no statistically significant differences in the frequencies of HLA-DRB1 alleles between TB patients and the HE group (data not shown). When the HE group was stratified for the presence of infection, however, we found that the HLA-DRB1*14 allele was absent in the HE+ group (0/6 cases) (7 % vs. 0; p = 0.038) (Table 2).
HLA-DRB1* alleles phenotype frequency in TB patients and HE+ individuals
| Alleles HLA-DRB1* | TB patients (n = 92) | HE+ (n = 69) | HE— | ||
| n (%) | n (%) | p value | n (%) | p value | |
| 1 | 21 (23) | 16 (23) | 3 (23) | ||
| 3 | 14 (15) | 15 (22) | 1 (8) | ||
| 4 | 27 (29) | 15 (22) | 3 (23) | ||
| 7 | 25 (27) | 22 (32) | 3 (23) | ||
| 8 | 13 (14) | 7 (10) | 1 (8) | ||
| 9 | 1 (1) | 2 (3) | 0 | ||
| 10 | 5 (5) | 1 (1) | 1 (8) | NS | |
| 11 | 15 (16) | 16 (23) | 4 (31) | ||
| 12 | 0 | 3 (4) | 0 | ||
| 13 | 23 (25) | 18 (26) | 3 (23) | ||
| 14 | 6 (7) | 0 | 0.038 | 1 (8) | |
| 15 | 17 (18) | 10 (14) | 4 (31) | ||
| 16 | 5 (5) | 7 (10) | 0 | ||
There were no statistically significant differences in the frequencies of the distribution of HLA-DQB1 alleles in TB patients and the HE group.
The allele frequencies for normal healthy individuals (HC) were not substantially different from those reported in the same region. 28
DiscussionTB pathogenesis is very complex, with many factors influencing disease development and determining the outcome of infection. MT usually enters the body via the respiratory route. Phagocytosis of MT by alveolar macrophages is the first event in the host-pathogen interaction and may determine the outcome of infection. Within 2 to 6 weeks of infection, cell-mediated immunity is developed and an influx of lymphocytes and activated macrophages appears in the lesion, resulting in granuloma formation. The bacilli are contained in the granuloma, where they may remain forever (latent TB infection), or become re-activated at a later date to proliferate and ultimately evolve to an active disease state.
Not all individuals exposed to MT become infected. Of those who do become infected, the course and duration of progression to active disease are highly variable. This lack of uniformity in disease manifestation among infected individuals may reflect a complex interaction between genetic and environmental factors. The relative weight of some risk factors, such as AIDS, diabetes, family income and nutritional status, are known, but host genetic factors may also influence susceptibility to infection and disease pathogenesis. Despite the compelling rationale for the involvement of host genetic influences, evidence for a genetic basis for TB susceptibility has been difficult to establish.
Some candidate genes have been reported, 15–18 and a role for HLA, which is important in the immune response, has been recognized. In particular, a number of studies have reported an association between HLA Class II alleles and TB, but these associations are not consistent across different populations 15–18 and the results remain controversial. A study by Ruggiero et al showed an increase in HLA-DR4 frequency in an Italian population. 29 A significant increase in the frequency of HLA-DRB1*14 has been reported in Iranian patients, 30 an association that was confirmed by Matrashkin et al in a Russian population. 31 A case-control study published by Dubaniewicz et al reported a strong association of HLA-DRB1*16 with TB in a Polish population. 32 More recently, this group confirmed and extended these results using a “high resolution” method, showing a high frequency of the HLA-DRB1*1601 allele 33 and a low frequency of HLA-DQB1*0201 in Polish TB patients; this latter result suggests that the HLA-DQB1*0201 allele may be linked to TB resistance. A high frequency of the HLA-DQB1*0501 allele has been reported among North American Indian 34 and Mexican 35 TB patients, whereas the frequency of the HLA-DQB1*0502 was increased among TB patients in a Thai population. 16 In a report from Cambodia, the HLA-DQB1*0503 allele was found to be associated with TB whereas the HLA-DQB1*0501 allele was not. 15 These differences are likely attributable to the differing ethnic backgrounds of the studied populations, but it should be stressed that some older studies used serological typing methods of dubious specificity and sensitivity.
In this study we analyzed the distribution of HLA-DRB1 and HLA-DQB1 alleles in 92TB patients and 82 disease-free healthcare professionals exposed to TB. The healthcare professionals chosen were from hospitals with a history of TB inpatient treatment and from ambulatory TB centers; only those individuals who had worked in these settings prior to 2000 were selected. Until very recently, there were no preventive measures against nosocomial TB in these settings except for individual protection. Most had worked in the same settings when the hospitals were sanatoria and exposure to TB was very high, a fact that explains the high prevalence of positive tuberculin skin test reactions (69/82) detected among these healthcare professionals.
There are two tests used in clinical practice to identify individuals with latent TB. These are the tuberculin skin test, and the interferon-gamma release assays (IGRAs), which identify a memory of an adaptive immune response against mycobacterial antigens. At the time this project was run, IGRAs were not widely available in our country so we could not use it.
The sensitivity of the tuberculin skin test is compromised in individuals with immunosuppression due to disease or treatment, but those were not studied here. The choice of TST cutpoint influences the probability that a positive TST reaction is a true positive due to M. tuberculosis versus a false positive, often due to cross-reactions from infection with nontuberculous mycobacteria (NTM), or BCG vaccination. But on the other side, NTM-induced TST reactions are generally in the 5 to 14-mm range. 36,37 All exposed group who tested positive had reactions over 15mm which is very likely MT- induced
We found that the HLA-DRB1*14 allele frequency is higher among TB patients compared to HE+ group, suggesting that HLA-DRB1*14 could be a susceptibility allele for TB disease. Although the difference is relatively small, it is in agreement with previous reports from Iran and Russia, where this allele was found to be increased among TB patients. Confirmation of these observations will require further study using larger groups of patients and healthy, TB-exposed individuals.
Conflict of interestAuthors state that they don't have any conflict of interest.