We determined the prevalences of hyperoxemia and excessive oxygen use, and the epidemiology, ventilation characteristics and outcomes associated with hyperoxemia in invasively ventilated patients with coronavirus disease 2019 (COVID–19).
MethodsPost hoc analysis of a national, multicentre, observational study in 22 ICUs. Patients were classified in the first two days of invasive ventilation as ‘hyperoxemic’ or ‘normoxemic’. The co–primary endpoints were prevalence of hyperoxemia (PaO2 > 90 mmHg) and prevalence of excessive oxygen use (FiO2 ≥ 60% while PaO2 > 90 mmHg or SpO2 > 92%). Secondary endpoints included ventilator settings and ventilation parameters, duration of ventilation, length of stay (LOS) in ICU and hospital, and mortality in ICU, hospital, and at day 28 and 90. We used propensity matching to control for observed confounding factors that may influence endpoints.
ResultsOf 851 COVID–19 patients, 225 (26.4%) were classified as hyperoxemic. Excessive oxygen use occurred in 385 (45.2%) patients. Acute respiratory distress syndrome (ARDS) severity was lowest in hyperoxemic patients. Hyperoxemic patients were ventilated with higher positive end–expiratory pressure (PEEP), while rescue therapies for hypoxemia were applied more often in normoxemic patients. Neither in the unmatched nor in the matched analysis were there differences between hyperoxemic and normoxemic patients with regard to any of the clinical outcomes.
ConclusionIn this cohort of invasively ventilated COVID–19 patients, hyperoxemia occurred often and so did excessive oxygen use. The main differences between hyperoxemic and normoxemic patients were ARDS severity and use of PEEP. Clinical outcomes were not different between hyperoxemic and normoxemic patients.
Both severe and moderate hyperoxemia have been reported to be associated with worse outcomes in critically ill patients,1,2 and high levels of fraction of inspired oxygen (FiO2) have detrimental effects on lung tissue, causing damage comparable to that seen in acute respiratory distress syndrome (ARDS).3,4 However, the exact targets of arterial oxygen tension (PaO2) and FiO2 remain debated, especially in ARDS patients where the relationship between oxygenation and outcome is complex. One seminal study, named ‘OXYGEN–ICU’, showed a conservative oxygen strategy targeting PaO2 of 70–100 mmHg compared with a liberal oxygenation therapy targeting PaO2 > 150 mmHg to improve survival.5 More recent studies comparing a conservative oxygen strategy with less liberal oxygen strategies, however, failed to show benefit.6-11
Coronavirus disease 2019 (COVID–19) is currently the most common form of ARDS, and patients with COVID–19 ARDS almost always experience profound impairments in gas exchange.12-14 To determine the exact prevalence of hyperoxemia and of excessive oxygen use in invasively ventilated COVID–19 patients, we performed a secondary analysis of a conveniently–sized multicentre observational study, named the ‘PRactice of VENTilation in COVID–19’ (PRoVENT–COVID).15 We compared the epidemiology, ventilation characteristics and outcomes in hyperoxemic versus normoxemic patients. We used propensity matching to control for observed confounding factors. The hypothesis was that hyperoxemia and excessive oxygen use occur often in COVID–19 patients under invasive ventilation.
MethodsStudy designSecondary analysis of PRoVENT–COVID, an investigator–initiated, national, multicentre, observational cohort study undertaken at 22 ICUs in the Netherlands. The study protocol of PRoVENT–COVID and the analysis plan for the current analysis have been prepublished16,17 and the study is registered at clinicaltrials.gov (NCT04346342). Other post–hoc evaluations of PRoVENT–COVID regarding ventilation characteristics and strategies,15,18,19 and gas exchange,20,21 were reported earlier.
PatientsConsecutive patients were eligible for participation if they were > 18 years of age, admitted to one of the participating ICUs, and had received invasive ventilation for acute hypoxemic respiratory failure related to COVID–19. COVID–19 was to be confirmed by RT–PCR. For the current analysis, we excluded patients that were transferred from or to a non–participating hospital in the first two days of invasive ventilation, as we could not collect data on gas exchange during these days in those patients, and patients without PaO2 data on the first two days of invasive ventilation.
Collected dataPatient demographics, medical history, presence and severity of ARDS, and extent of infiltrates on the chest radiography or computed tomography scan was collected at baseline.
Since the first day of ventilation had a flexible length and could range from one minute to 24 hours duration depending on the timing of start of invasive ventilation in the ICU, we merged this day with the second day and named it ‘day 1’. The following calendar day was named ‘day 2’.
We collected detailed ventilation data at one hour after start of invasive ventilation in the ICU, which could be at arrival if the patients started with invasive ventilation in the normal ward or in the emergency room, or after intubation in the ICU after ICU admission. Thereafter, we collected ventilation data at 08:00, 16:00 and 24:00 hours over the first four days of ventilation. Ventilation data included ventilator settings and ventilation parameters, arterial blood gas analyses results and use of adjunctive therapies for refractory hypoxemia.
We also collected typical aspects of ICU monitoring and care, and common ICU complications. Patients were followed until day 90 for intubation status, ICU– and hospital–discharge, and death.
ExposuresThe primary exposure of interest was hyperoxemia on day 1 or day 2 of invasive ventilation, defined as PaO2 > 90 mmHg. Patients were categorized as ‘hyperoxemic’ if the daily mean PaO2 was > 90 mmHg, or ‘hypoxemic’ if the daily mean PaO2 was ≤ 55 mmHg, on either day 1 or day 2; all other patients were classified as ‘normoxemic’. The first PaO2 value was ignored, because it is plausible that this value could not be affected by FiO2 titrations by ICU team members.
The secondary exposure of interest was excessive use of oxygen. At each time–point on day 1 and day 2, oxygen use was classified as ‘excessive’ if FiO2 was ≥ 60% following a previous blood gas analysis showing PaO2 > 90 mmHg or recorded SpO2 > 92%.
OutcomesThe co–primary outcomes were the prevalence of hyperoxemia and the prevalence of excessive oxygen use. Secondary outcomes included key ventilator settings and parameters, including tidal volume (VT), positive end–expiratory pressure (PEEP), driving pressure (ΔP) and respiratory system compliance (Crs), and typical clinical outcomes, including duration of ventilation, length of stay (LOS) in hospital and ICU, and mortality in ICU, hospital, and at day 28 and day 90.
Statistical analysisDue to the very small number of hypoxemic patients, i.e., 8 out of 851 patients, hypoxemic patients were added to the cohort of normoxemic patients in all analyses.
To assess differences between hyperoxemic and normoxemic patients, Wilcoxon–Mann–Whitney test for continuous data and Fisher exact test for categorical data were used. Ventilation data was reported at three specific moments: 1) at start of ventilation, 2) on day 1, and 3) on day 2. Start of ventilation was based on the measurements collected within the first hour after start of ventilation. As previously mentioned, the measurements of the first flexible calendar day and first full calendar day were merged, and day 1 was based on the means of these measurements. Day 2 was based on the means of the measurements of the following calendar day. Cumulative frequency distributions of VT, PEEP, ΔP, and Crs are shown for patients categorized as hyperoxemic versus patients that are categorized as normoxemic, at day 1 and at day 2. Locally estimated scatterplot smoothing (LOESS) method was used to inspect the relationship between 28–day mortality and PaO2 and FiO2 at day 1 and at day 2.
To further evaluate the associations of outcome with occurrence of hyperoxemia, a propensity matched analysis was performed. For each patient, a propensity score was estimated with logistic regression and used to match hyperoxemic patients to normoxemic patients (1:1) using a caliper of 0.05 standard deviation of the logit of the propensity score and applying nearest matching without replacement. Based on clinical relevance and one previous analysis,22 the following variables were selected a priori: age, sex, BMI, chronic diseases including heart failure, diabetes mellitus, chronic renal failure, chronic liver failure, chronic pulmonary obstructive disease, active or hematologic neoplasm and immunosuppression, and PaO2/FiO2, Crs, total respiratory rate (RR) and bicarbonate at baseline.
Time until extubation is shown in a cumulative distribution plot with death as a competing risk and compared with a Fine–Gray competing risk model. Probability of survival at day 28 and 90 was estimated using Kaplan–Meier curves and compared with a log–rank test.
In a sensitivity analysis, we excluded the hypoxemic patients to check whether there were differences in clinical outcomes.
All analyses were conducted in R v.3.6.1 (R Foundation, Vienna, Austria) and significance level was set at 0.05.
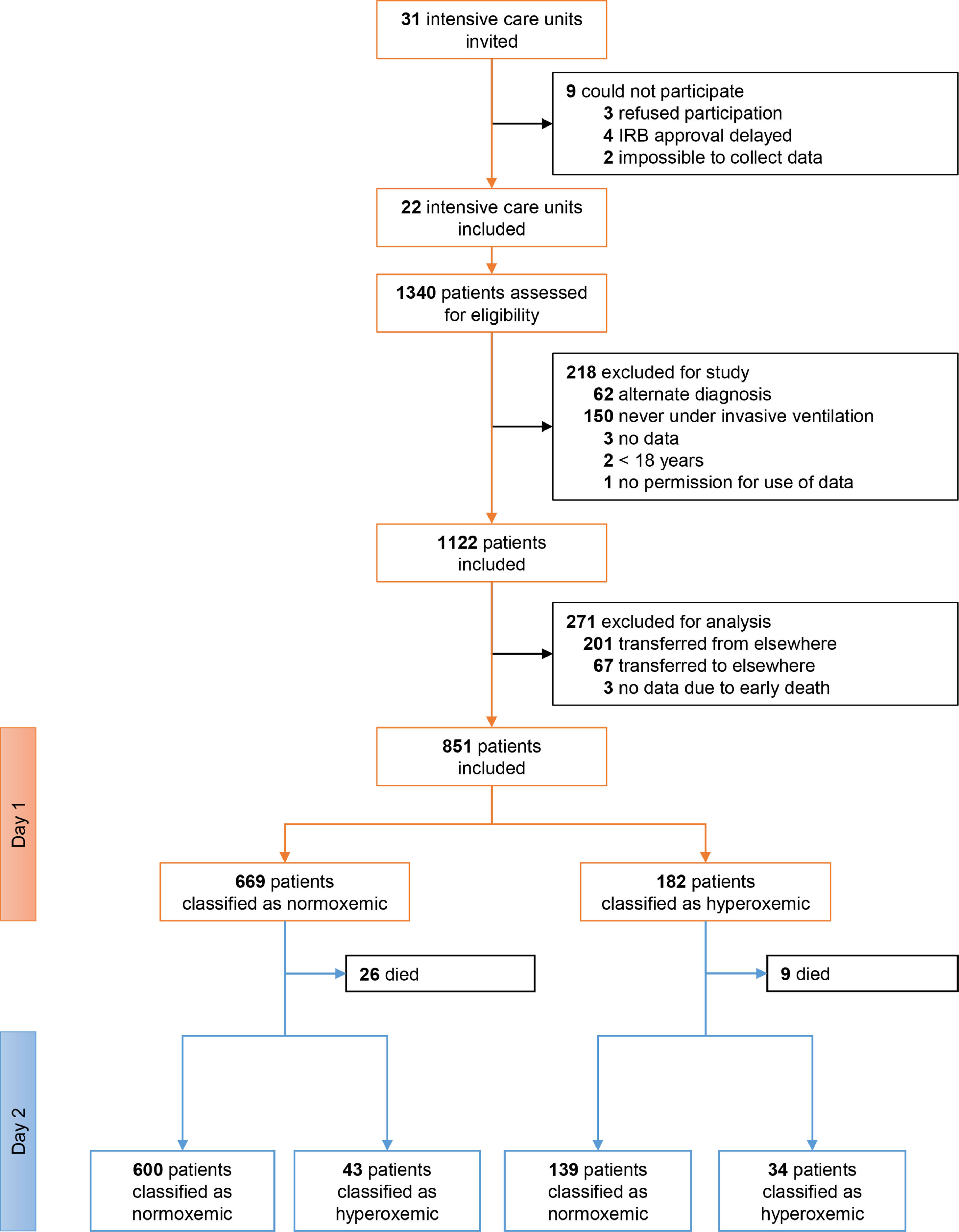
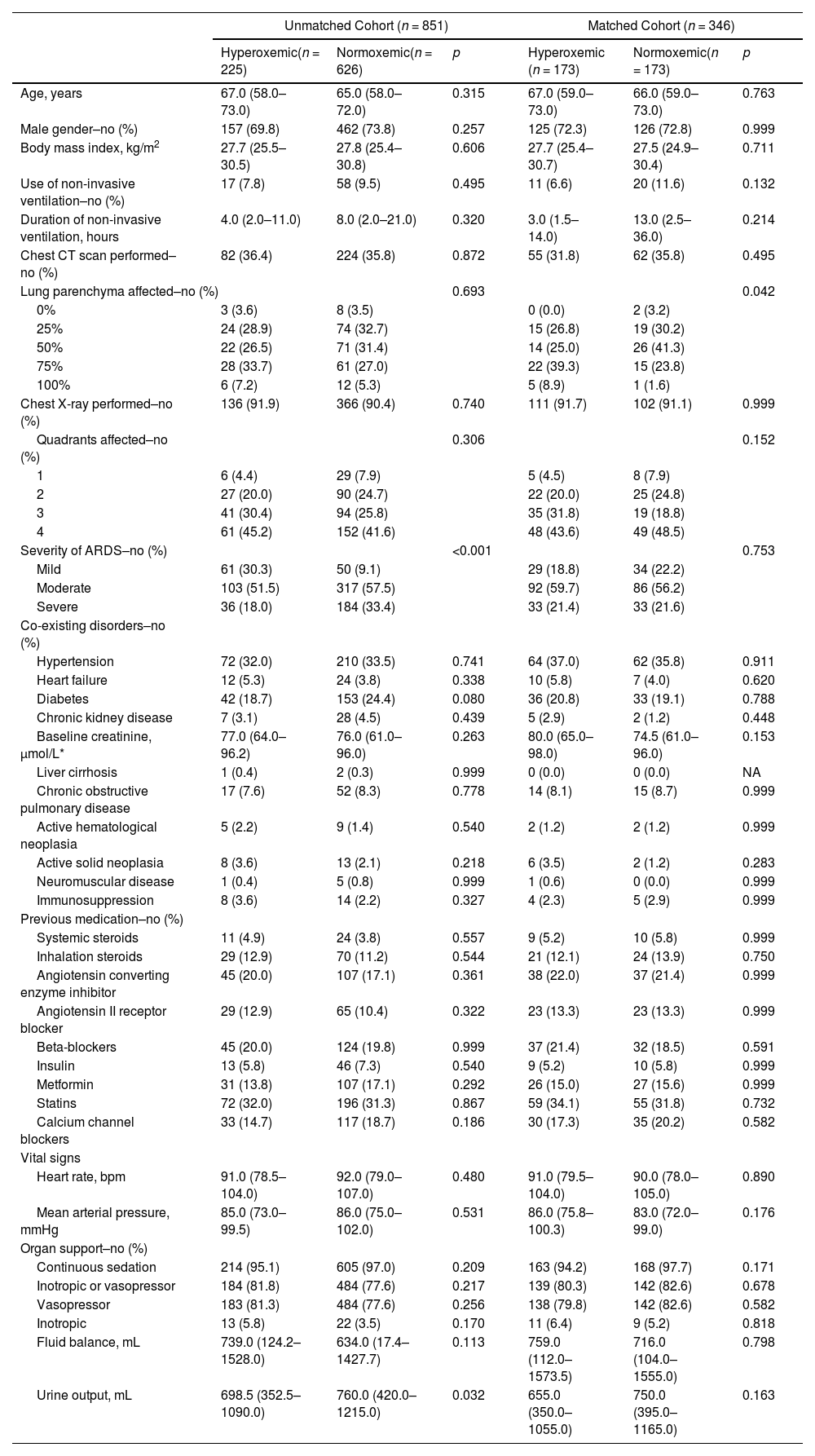
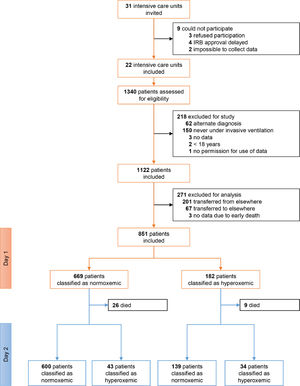
ResultsPatients1122 patients were included in PRoVENT–COVID (Fig. 1). We excluded 271 patients, mainly because of early transfer from or to a non–participating ICU. The remaining 851 patients most often were male (73%) with a median age of 66 [58–72] years, and the majority of patients had moderate to severe ARDS (Table 1).
Baseline Characteristics of the Patients According to the Groups In the Unmatched and Matched Cohort.
Data are median (quartile 25% - quartile 75%) or No (%). Percentages may not total 100 because of rounding.
Of 851 patients, 182 (21.4%) patients were hyperoxemic on day 1 and 77 (9.0%) patients were hyperoxemic on day 2. Only 34 (4%) patients were hyperoxemic on both days, but 225 (26.4%) patients were hyperoxemic on either day 1 or day 2 (Fig. 1). eTable 1, eFigure 1 and eResults show group assignments, and daily mean PaO2 and SpO2.
Excessive oxygen use occurred at least once in 385 (45.2%) patients. The prevalence was not different between hyperoxemic and normoxemic patients on day 1 but occurred more often in normoxemic patients on day 2 (eTable 2). eTable 1, eFigure 1 and 2, and eResults show group assignments, and daily mean FiO2. Median daily FiO2 was slightly lower in hyperoxemic patients on both days.
Patient demographics and ventilation parametersModerate to severe ARDS was less often seen in hyperoxemic patients than in normoxemic patients, and hyperoxemic patients had a lower urinary output and a slightly higher plasma lactate at baseline (Table 1).
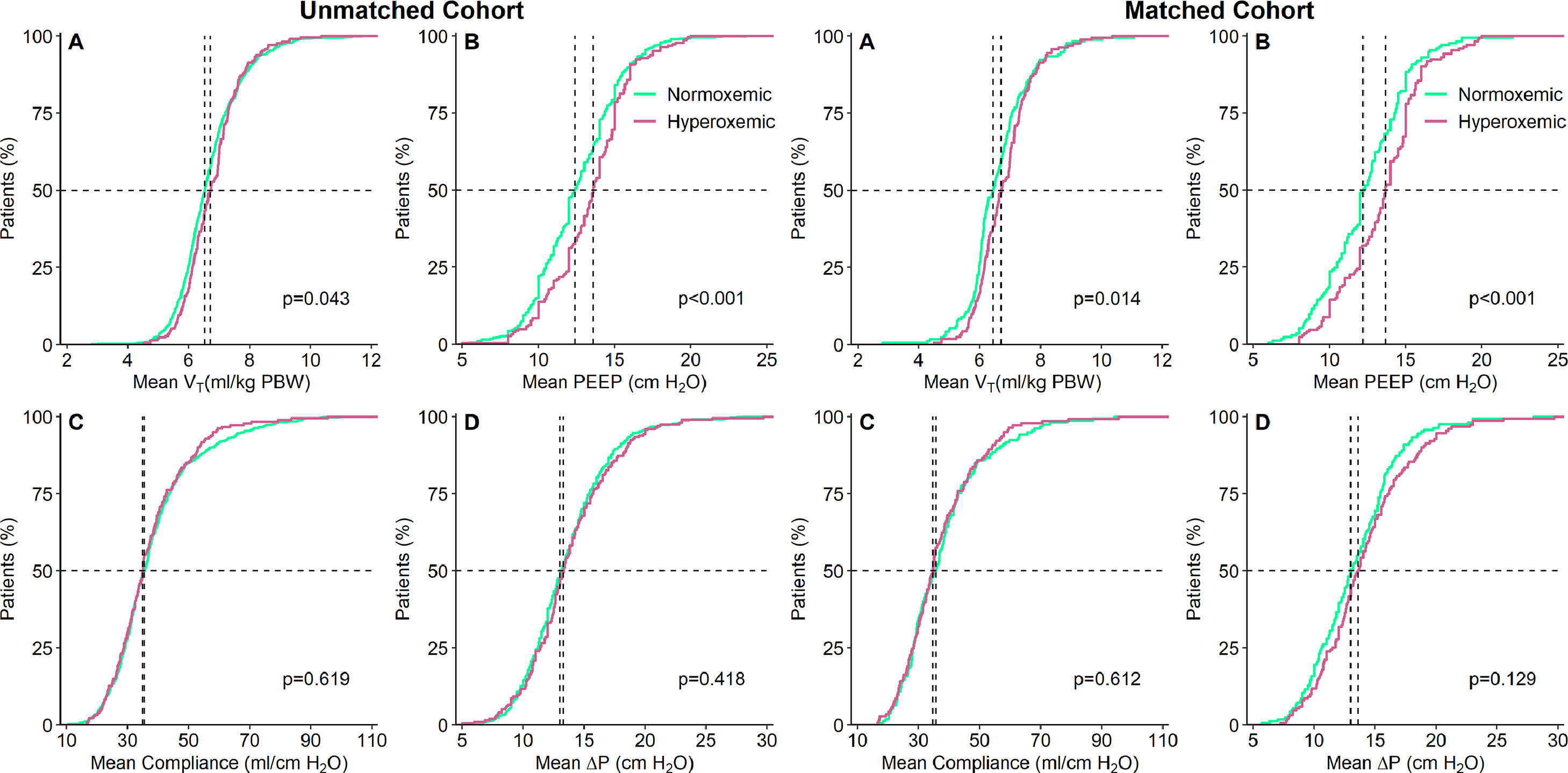
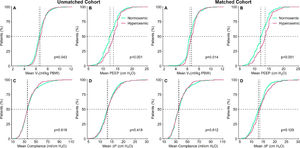
At start of ventilation, hyperoxemic patients received a similar VT at a comparable ΔP, and consequently had comparable Crs to normoxemic patients (eTable 1). At start of ventilation, hyperoxemic patients received ventilation with higher PEEP than normoxemic patients. VT was slightly higher in hyperoxemic patients, and the difference in PEEP persisted over the successive days (Fig. 2, eTable 2 and eFigure 3). Rescue therapies for hypoxemia were applied more often in normoxemic patients (Table 2 and eTable 2).
Cumulative frequency distributions of ventilation variables on day 1 of ventilation in the hyperoxemic (purple) and normoxemic (green) group, in the unmatched (left panels) and matched (right panels) cohorts. Horizontal dotted lines represent 50% of the patients and vertical dotted lines represent the median of the variable at the start of ventilation. All measurements are the means of a maximum of six measurements. P–values from Wilcoxon–Mann–Whitney test.
VT: tidal volume; PBW: predicted body weight; MP: mechanical power; VR: ventilator ratio; ΔP: driving pressure, RR: respiratory rate; MV: minute ventilation.
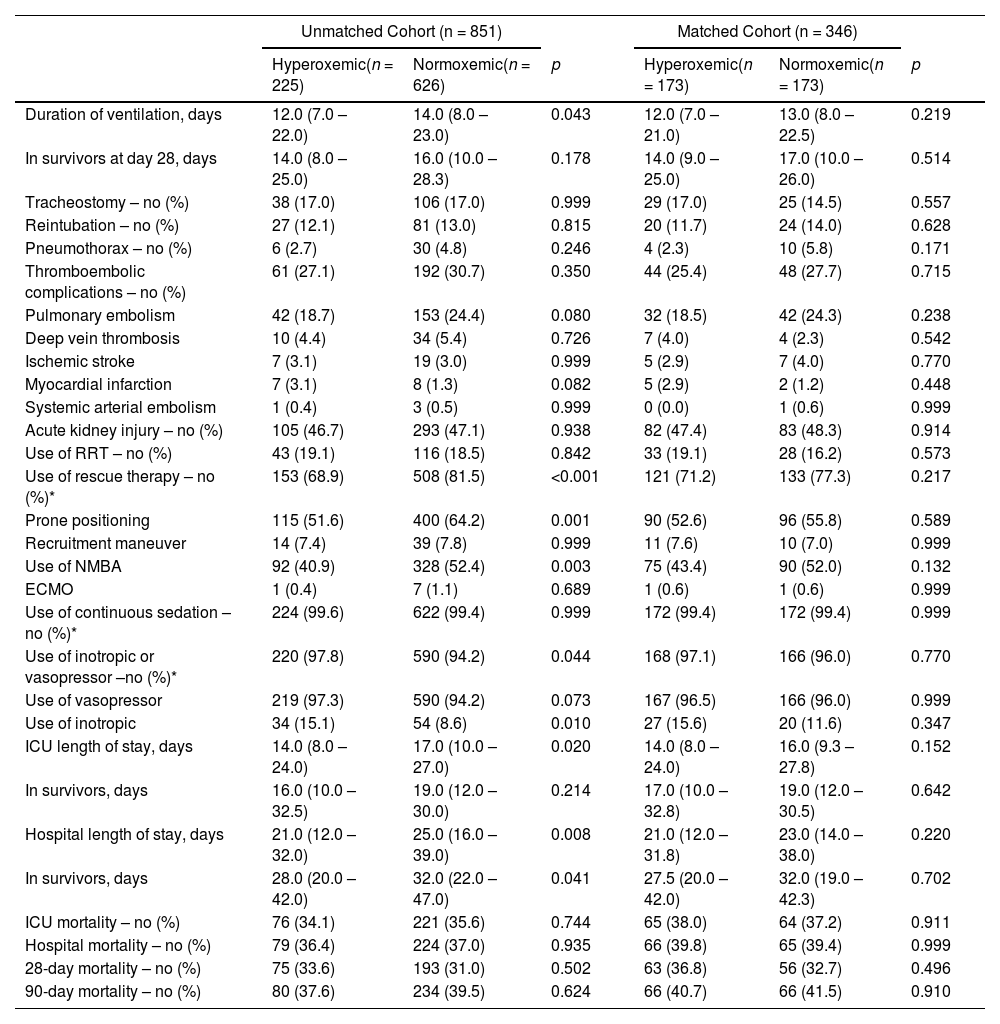
Clinical Outcomes According to Groups In the Unmatched and Matched Cohort.
| Unmatched Cohort (n = 851) | Matched Cohort (n = 346) | |||||
|---|---|---|---|---|---|---|
| Hyperoxemic(n = 225) | Normoxemic(n = 626) | p | Hyperoxemic(n = 173) | Normoxemic(n = 173) | p | |
| Duration of ventilation, days | 12.0 (7.0 – 22.0) | 14.0 (8.0 – 23.0) | 0.043 | 12.0 (7.0 – 21.0) | 13.0 (8.0 – 22.5) | 0.219 |
| In survivors at day 28, days | 14.0 (8.0 – 25.0) | 16.0 (10.0 – 28.3) | 0.178 | 14.0 (9.0 – 25.0) | 17.0 (10.0 – 26.0) | 0.514 |
| Tracheostomy – no (%) | 38 (17.0) | 106 (17.0) | 0.999 | 29 (17.0) | 25 (14.5) | 0.557 |
| Reintubation – no (%) | 27 (12.1) | 81 (13.0) | 0.815 | 20 (11.7) | 24 (14.0) | 0.628 |
| Pneumothorax – no (%) | 6 (2.7) | 30 (4.8) | 0.246 | 4 (2.3) | 10 (5.8) | 0.171 |
| Thromboembolic complications – no (%) | 61 (27.1) | 192 (30.7) | 0.350 | 44 (25.4) | 48 (27.7) | 0.715 |
| Pulmonary embolism | 42 (18.7) | 153 (24.4) | 0.080 | 32 (18.5) | 42 (24.3) | 0.238 |
| Deep vein thrombosis | 10 (4.4) | 34 (5.4) | 0.726 | 7 (4.0) | 4 (2.3) | 0.542 |
| Ischemic stroke | 7 (3.1) | 19 (3.0) | 0.999 | 5 (2.9) | 7 (4.0) | 0.770 |
| Myocardial infarction | 7 (3.1) | 8 (1.3) | 0.082 | 5 (2.9) | 2 (1.2) | 0.448 |
| Systemic arterial embolism | 1 (0.4) | 3 (0.5) | 0.999 | 0 (0.0) | 1 (0.6) | 0.999 |
| Acute kidney injury – no (%) | 105 (46.7) | 293 (47.1) | 0.938 | 82 (47.4) | 83 (48.3) | 0.914 |
| Use of RRT – no (%) | 43 (19.1) | 116 (18.5) | 0.842 | 33 (19.1) | 28 (16.2) | 0.573 |
| Use of rescue therapy – no (%)* | 153 (68.9) | 508 (81.5) | <0.001 | 121 (71.2) | 133 (77.3) | 0.217 |
| Prone positioning | 115 (51.6) | 400 (64.2) | 0.001 | 90 (52.6) | 96 (55.8) | 0.589 |
| Recruitment maneuver | 14 (7.4) | 39 (7.8) | 0.999 | 11 (7.6) | 10 (7.0) | 0.999 |
| Use of NMBA | 92 (40.9) | 328 (52.4) | 0.003 | 75 (43.4) | 90 (52.0) | 0.132 |
| ECMO | 1 (0.4) | 7 (1.1) | 0.689 | 1 (0.6) | 1 (0.6) | 0.999 |
| Use of continuous sedation – no (%)* | 224 (99.6) | 622 (99.4) | 0.999 | 172 (99.4) | 172 (99.4) | 0.999 |
| Use of inotropic or vasopressor –no (%)* | 220 (97.8) | 590 (94.2) | 0.044 | 168 (97.1) | 166 (96.0) | 0.770 |
| Use of vasopressor | 219 (97.3) | 590 (94.2) | 0.073 | 167 (96.5) | 166 (96.0) | 0.999 |
| Use of inotropic | 34 (15.1) | 54 (8.6) | 0.010 | 27 (15.6) | 20 (11.6) | 0.347 |
| ICU length of stay, days | 14.0 (8.0 – 24.0) | 17.0 (10.0 – 27.0) | 0.020 | 14.0 (8.0 – 24.0) | 16.0 (9.3 – 27.8) | 0.152 |
| In survivors, days | 16.0 (10.0 – 32.5) | 19.0 (12.0 – 30.0) | 0.214 | 17.0 (10.0 – 32.8) | 19.0 (12.0 – 30.5) | 0.642 |
| Hospital length of stay, days | 21.0 (12.0 – 32.0) | 25.0 (16.0 – 39.0) | 0.008 | 21.0 (12.0 – 31.8) | 23.0 (14.0 – 38.0) | 0.220 |
| In survivors, days | 28.0 (20.0 – 42.0) | 32.0 (22.0 – 47.0) | 0.041 | 27.5 (20.0 – 42.0) | 32.0 (19.0 – 42.3) | 0.702 |
| ICU mortality – no (%) | 76 (34.1) | 221 (35.6) | 0.744 | 65 (38.0) | 64 (37.2) | 0.911 |
| Hospital mortality – no (%) | 79 (36.4) | 224 (37.0) | 0.935 | 66 (39.8) | 65 (39.4) | 0.999 |
| 28-day mortality – no (%) | 75 (33.6) | 193 (31.0) | 0.502 | 63 (36.8) | 56 (32.7) | 0.496 |
| 90-day mortality – no (%) | 80 (37.6) | 234 (39.5) | 0.624 | 66 (40.7) | 66 (41.5) | 0.910 |
Data are median (quartile 25% - quartile 75%) or No (%). Percentages may not total 100 because of rounding.
RRT: renal replacement therapy; NMBA: neuromuscular blocking agent; ECMO: extracorporeal membrane oxygenation.
A flat relationship was seen between 28–day mortality and PaO2 at day 1; a U–shape relationship was seen between 28–day mortality and PaO2 at day 2, with a nadir PaO2 of 75 mmHg (eFigure 4). Mortality rates increased with higher FiO2 (eFigure 5).
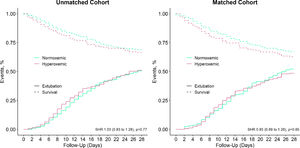
Duration of ventilation and length of stay in hospital and ICU were shorter in hyperoxemic patients (Table 2), but not when death was treated as a competing risk (Fig. 3, eFigure 6 and 7). Mortality was not different between the two groups (Table 2, eFigure 8 and 9).
Matched analysisWe matched 346 patients, resulting in fairly comparable groups, with persisting differences in PaO2 (Table 1, eTable 1 and 3, and eFigure 1, 10 and 11). In the matched analysis, there were no differences in any of the clinical outcomes (Table 2, Fig. 3, eFigures 6 to 9).
Sensitivity analysisThe sensitivity analysis in which we excluded hypoxemic patients did not show differences in clinical outcomes (eTable 4 and 5; eFigure 12 to 14).
DiscussionIn this cohort of intubated patients with COVID–19 ARDS (1) the prevalence of hyperoxemia was high, and (2) many patients experienced excessive oxygen use, albeit that the prevalence per patient was low; in addition, (3) hyperoxemic patients received ventilation with a slightly higher VT, higher PEEP but a lower FiO2; and (4) there were no differences in clinical outcomes between hyperoxemic and normoxemic patients.
This analysis is one of the first to investigate the prevalence of hyperoxemia and excessive use of oxygen in a large cohort of invasively ventilated COVID–19 patients. Granular ventilation data was collected over the first days by investigators that were trained in data collection to ensure good quality of the data. Patients were included within a relatively short timeframe and therefore unlikely subjected to changes in the local protocols. We recruited patients in different types of hospitals, increasing the generalizability of the findings. This planned analysis was unknown to the caregivers at the time of data collection, minimizing the risk of observation bias. Finally, we had a sophisticated pre–published statistical analysis plan, including a propensity matched analysis to control for confounding factors.
The prevalence of hyperoxemia in our study was comparable,23,24 but mostly lower12,25,26 than in other cohorts of COVID–19 patients. One study that specifically examined hyperoxemia in invasively ventilated COVID–19 patients reported a prevalence threefold higher than in our cohort.26 Patients in that study, alike the other studies that reported a higher prevalence of hyperoxemia under invasive ventilation, had a higher PaO2/FiO2 at start of ventilation, suggesting that patients in those studies had less severe ARDS. It cannot be excluded, however, that the caregivers involved in care for patients in our cohort targeted lower oxygen levels, either because of the local protocols that were being used, or because they are more aware of the potential risks of hyperoxemia.27-29
The prevalence of hyperoxemia was remarkably lower than that seen in a large international cohort of ARDS patients from 2014.22 This difference may be explained in several ways. First, it is possible that ventilation strategies have changed over recent years. Lower tidal volumes are increasingly used, and VT reduction can result in lower oxygen levels, as also seen in the seminal ARDS Network trial named ‘ARMA’.30 Second, and in line with the suggestion above on oxygen targets, the findings of several studies in this topic5,29,31 may have resulted in lower oxygen targets. Third, hyperoxemia could be more difficult to achieve in patients with COVID–19 ARDS, due to extensive pulmonary infiltrates or sometimes the presence of pulmonary embolism.
One important finding of our study is that outcomes were not different between hyperoxemic versus normoxemic patients. However, this may not be too surprising since the differences in oxygen levels between the two patient groups were not as large as in the initial investigations that studied the effects of hyperoxemia in critically ill patients––of note, this was also the case in the recent randomized clinical trials that all showed no benefit of a low oxygen versus a high oxygen strategy.8-11 However, we noted an increasing mortality beyond a PaO2 of 75 mmHg in the LOESS curve.
Differences in ventilation between hyperoxemic and normoxemic patients were minimal. PEEP, however, was consistently higher in the hyperoxemic patients, even after matching. This may not be unexpected, as higher PEEP can result in more lung recruitment and thus improve oxygenation, as for instance also seen in patients in one study that was performed before just before the COVID–19 pandemic.32 Alike in patients with ARDS due to another cause, PEEP can also reaerate consolidated regions of the lungs in COVID–19 patients,33 thereby improving oxygenation.34 However, we do not suggest that high PEEP should be applied in all COVID–19 patients to improve oxygenation, as improved oxygenation does not automatically result in better outcome and non–recruitable patients are at high risk of overdistension and hemodynamic impairment.32 Interestingly, prone positioning was used more often in the normoxemic group. This finding suggests that prone positioning was adequately used as a rescue therapy for refractory hypoxemia.
Excessive oxygen use was seen in almost half of the patients in this cohort. However, only 13.6% of all the observed time–points showed excessive use of oxygen, which at least suggests that clinicians responded adequately to hyperoxemia with a reduction in FiO2. Interestingly, use of excessive oxygen was much lower than in previous cohorts of patients with ARDS due to another cause.22,27,35 Of note, the LOESS curve demonstrated increasing mortality with increasing levels of FiO2 regardless of the presence of hyperoxemia, and although this is partially linked to severity of disease, it could also represent the deleterious effects that high levels of FiO2 can have on lung tissue.36
Limitations of this analysis are as follows. We restricted the analysis to the first two days of oxygenation, and therefore cannot be sure whether dysoxemia at later time points had detrimental effects. The definitions of hyperoxemia and excessive oxygen were arbitrary, as there are no definitive targets for PaO2 or FiO2. Higher cut-offs for PaO2 and FiO2 could have resulted in other prevalences of hyperoxemia and excessive oxygen use, and maybe even associations of dysoxemia with outcome. Due to the low number of hypoxemic patients in this cohort, we were not able to compare outcomes in these patients. However, the sensitivity analysis in which we excluded hypoxemic patients showed that there were no differences in clinical outcomes. We were not able to collect data to gain insight in oxygen management per se, and we did also not collect the protocols in place at the participating centres. Unfortunately, we did not acquire ventilation data before intubation and therefore were not able to calculate the ROX index in patients previously on high flow nasal oxygen (HFNO). This index (ratio of SpO2/FiO2 to respiratory rate (RR)) predicts whether patients on HFNO will be in need of intubation, and it would have been interesting to see whether hyperoxemic patients had a lower chance of HFNO failure based on the ROX index.
ConclusionsIn this cohort of COVID–19 ARDS patients, hyperoxemia and excessive oxygen use occurred often, but prevalences were lower than in previous studies in patients with ARDS due to another cause. The main difference between hyperoxemic and normoxemic patients was ARDS severity, use of PEEP and FiO2 and prone positioning. We found no effect of hyperoxemia on outcomes.
Ary Serpa Neto discloses personal fees received from Drager, outside the submitted work. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
PRoVENT–COVID Collaborative Group: (in alphabetic order): S. Ahuja; J.P. van Akkeren; A.G. Algera; C.K. Algoe; R.B. van Amstel; P. van de Berg; D.C. Bergmans; D.I. van den Bersselaar; F.A. Bertens; A.J. Bindels; J.S. Breel; C.L. Bruna; M.M. de Boer; S. den Boer; L.S. Boers; M. Bogerd; L.D. Bos; M. Botta; O.L. Baur; H. de Bruin; L.A. Buiteman–Kruizinga; O. Cremer; R.M. Determann; W. Dieperink; J. v. Dijk; D.A. Dongelmans; M.J. de Graaff; M.S. Galekaldridge; L.A. Hagens; J.J. Haringman; S.T. van der Heide; P.L. van der Heiden; L.L. Hoeijmakers; L. Hol; M. W. Hollmann; J. Horn; R. van der Horst; E.L. Ie; D. Ivanov; N.P. Juffermans; E. Kho; E.S. de Klerk; A.W. Koopman; M. Koopmans; S. Kucukcelebi; M.A. Kuiper; D.W. de Lange; I. Martin–Loeches; G. Mazzinari; D.M. van Meenen; N. van Mourik; S.G. Nijbroek; E.A. Oostdijk; F. Paulus; C.J. Pennartz; J. Pillay; I.M. Purmer; T.C. Rettig; O. Roca; J.P. Roozeman; M.J. Schultz; A. Serpa Neto; G.S. Shrestha; M.E. Sleeswijk; P.E. Spronk; A.C. Strang; W. Stilma; P. Swart; A.M. Tsonas; C.M.A. Valk; A.P. Vlaar; L.I. Veldhuis; W.H. van der Ven; P. van Velzen; P. van Vliet; P. van der Voort; L. van Welie; B. van Wijk; T. Winters; W.Y. Wong; A.R. van Zanten.