To evaluate the impact of positive airway pressure (PAP) therapy on body mass index (BMI) in patients with obesity hypoventilation syndrome (OHS) associated with obstructive sleep apnea (OSA). Methods: A systematic review using the following terms: “obesity hypoventilation syndrome” AND “treatment” AND “randomized” using Cochrane Central Register of Controlled Trials, Medline and Web of Science was performed from the first data available until February 10, 2023. The inclusion criteria were: (1) original article; (2) adult OHS with concomitant OSA (apnea-hypopnea index or AHI ≥5 events/h); (3) randomized trial with PAP arm and standard care (control); (4) BMI evaluation at baseline and after the first months. We performed an individual participant data meta-analysis of randomized controlled trials.
ResultsOur initial search retrieved 32 articles and 3 randomized studies fulfilled study criteria and were included in the final analysis, leading to a total of 342 participants. Patients were predominantly females (62%) and had OHS associated with at least mild OSA. As compared to baseline, a decrease in BMI was observed at study endpoint but this difference was not different intergroups (-0.50 ± 1.49 and -0.50 ±1.83, in control and PAP groups respectively (p=0.939)). Weight change was not associate with PAP adherence, OSA severity or use of supplemental oxygen.
ConclusionsIn contrast to treatment of eucapnic OSA with PAP that is associated with weight gain, treatment of OSA+OHS patients with or without PAP is associated with weight loss. Future studies are necessary to elucidate the mechanism by which weight loss occurs.
Current Knowledge/Study Rationale: Obesity hypoventilation syndrome (OHS) and obstructive sleep apnea (OSA) frequently co-exist, and positive airway pressure (PAP) is the treatment of choice. PAP is associated with weight gain in patients with eucapnic OSA. Since obesity plays a key role in the pathogenesis of OHS, the impact of PAP therapy on weight in patients with OSA+OHS must be clarified.
Study Impact: This individual participant data meta-analysis of randomized trials that included arms with and without PAP, showed that management of OSA+OHS is associated with weight loss, independent of PAP therapy.
IntroductionObesity is a major problem in modern society. In recent decades, the prevalence of obesity and severe obesity (Class III or BMI ≥40 kg/m2) has increased worldwide.1 The Centers for Disease Control and Prevention estimated that 7.6% of the adult United States population has a BMI ≥40 kg/m2 . Of all the complications related to morbid obesity, the development of ventilatory failure is one of the most serious, with a fourfold increase in hospitalization rates and increased mortality if untreated.2 Obesity hypoventilation syndrome (OHS) is defined by presence of obesity (BMI ≥30 kg/m2), hypercapnia with partial pressure of CO2 in the arterial blood (PaCO2) ≥45 mmHg during wakefulness, and sleep-disordered breathing in the absence of other diseases causing daytime hypoventilation.2-4 The prevalence of OHS in the general adult population has been estimated between 0.3 to 0.4%.5 However, in a population of morbidly obese patients, 23% were found to be hypercapnic.6 OHS is associated with poor quality of life, cardiovascular and metabolic co-morbidities and high mortality.7,8 Approximately 90% of patients with OHS have concomitant obstructive sleep apnea (OSA), with 70% having severe OSA, defined by an apnea-hypopnea index (AHI) ≥30 events/h.9
Positive airway pressure (PAP) during sleep is the gold standard therapy for OHS and can be applied either in the form of continuous positive airway pressure (CPAP) or bilevel PAP therapy. CPAP is recommended as the initial treatment for ambulatory patients with OHS and concomitant severe OSA.10,11 Both PAP modalities can improve ventilatory failure, quality of life and awake PaCO2 among ambulatory patients with OHS.10,12,13 On the other hand, there is consistent evidence that the treatment of eucapnic OSA with CPAP induces weight gain.14-16 This unintended effect in patients with eucapnic OSA can be particularly concerning if it also occurs among patients with OSA+OHS, a population in which obesity has led to chronic hypercapnic respiratory failure. The mechanism leading to weight gain during OSA treatment with CPAP is not fully understood. We have recently shown that weight gain occurs within one week of treatment and is associated with fluid retention.15 In this study we hypothesized that the treatment of OSA+OHS would be associated with weight loss. The hypothesis was driven by the rationale that mechanisms related to fluid retention such as persistent hypoxemia and pulmonary hypertension are ameliorated with OHS treatment.17 To test this hypothesis, we performed a systematic review and individual participant data meta-analysis of all randomized controlled trials that had a treatment arm with PAP and a control arm without PAP in patients with OSA+OHS. Using individualized data, we compared BMI before and after treatment with and without PAP therapy.
MethodsLiterature searchA systematic review was initially performed according to the Cochrane Collaborative group recommendations and the Preferred Reporting Items for Individual Participant Data systematic reviews and meta-analysis (PRISMA-IPD).18 One of the authors (RGSA) identified studies using the following terms: “obesity hypoventilation syndrome” AND “treatment” AND “randomized” in Cochrane Central Register of Controlled Trials, Medline and Web of Science. Case reports, case series, cohort studies, systematic reviews and meta-analyses were excluded. Retrieved articles were screened for relevant data. No language or time restrictions were applied. The search was performed from the first data available until February 10, 2023. Authors were contacted to provide individualized data for all patients and additional information when data was important for the present study was missing. Discrepancies were resolved by discussion with another investigator. This Individual Participant Data meta-analysis was registered in PROSPERO database with the number CRD 4202238172.
Quality assessmentThe methodological quality of each trial was assessed using internal validity, i.e., “risk of bias (RoB 2.0)” as recommended by the Cochrane Collaboration.19-20
Inclusion criteriaPotential published articles were identified and screened by their title and abstract. Full article from selected articles were retrieved. The inclusion criteria were: (1) randomized-controlled trial design; (2) adult OHS with concomitant OSA (AHI ≥5 events/h); (3) randomized trial with PAP arm and standard care (control); (4) BMI evaluation before and after the first months of enrollment in the study.
Data extractionData were extracted by an independent investigator and included the last name of the first author, year of publication, country, inclusion criteria, sample size, patient characteristics, length of follow up with PAP therapy. The first author of the selected paper was contacted, and individual data of the patients were obtained.
Statistical analysisData was described as mean±SD or median [interquartile range] after testing for normal distribution (Kolmogorov-Smirnov Test). We excluded participants with extreme outlying values of Δ BMI (>4 SDs) in order to eliminate probable typographical errors or effects of unrelated health issues and to ensure a consistent case base for all analyses as previously reported.19 To compare the difference between quantitative variables we used the Mann-Whitney test and for qualitative variables we used the Pearson Chi Square test. The generalized estimating equations (GEE) of individual data was used to compare changes in BMI according to assigned group (control and PAP) at baseline and at the end of the study. The subgroups explored were presence or absence of: heart failure, severe OSA (AHI ≥ 30 events/hour), hypoxemia and hypercapnia at baseline (divided by median levels of baseline PaO2 and PaCO2); oxygen not added or added to PAP, poor or adequate PAP adherence (≥ 4h/night). The model adjustment was evaluated by the quasi-likelihood under the Independence model criterion (QIC) and corrected quasi-likelihood under the Independence model criterion (QICC). Under these criteria, lower values of QIC and QICC indicate better fit of the model. All analyses were performed using the statistical package IBM SPSS Statistics version 25©. A P value of < 0.05 was considered statistically significant.
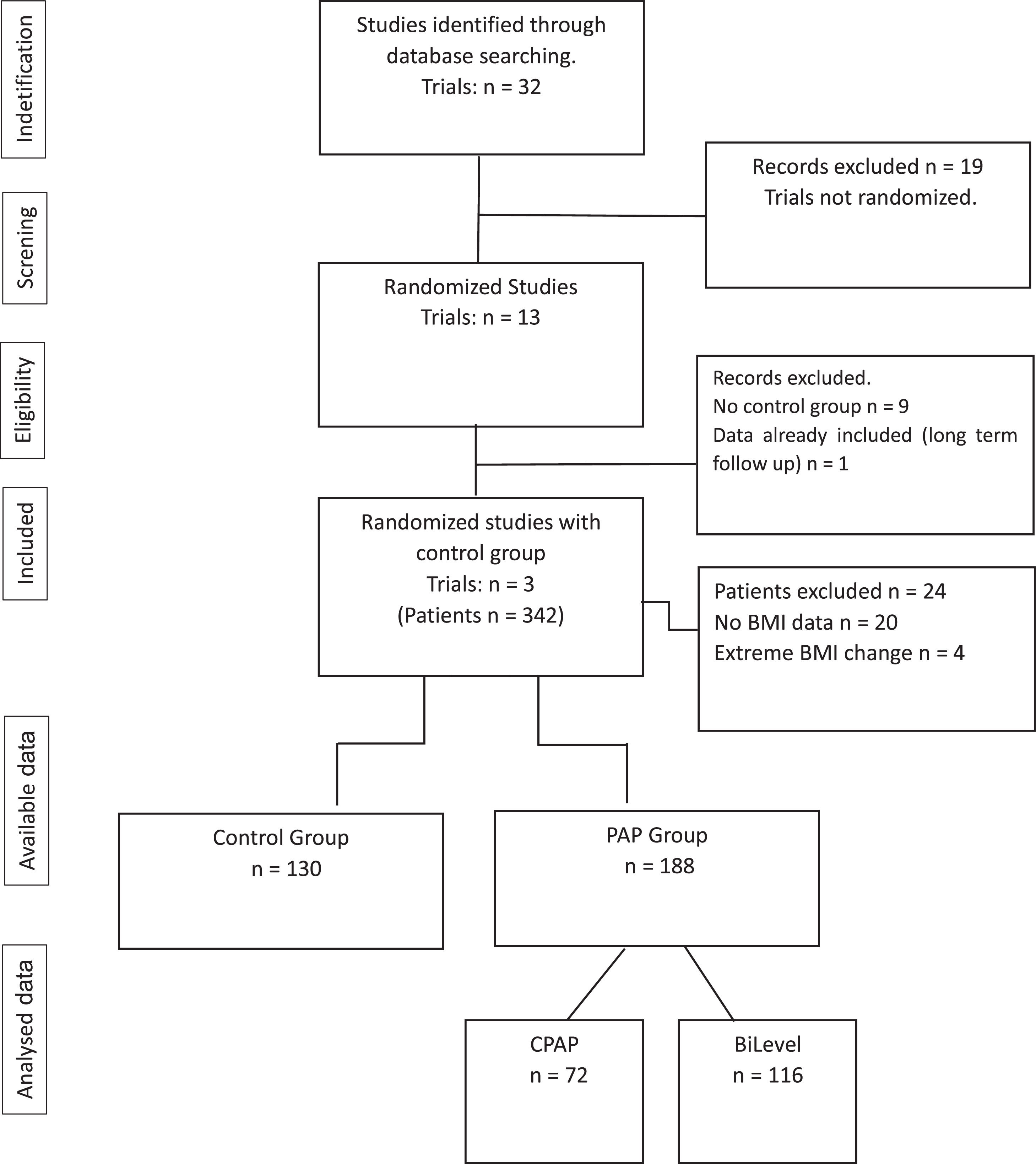
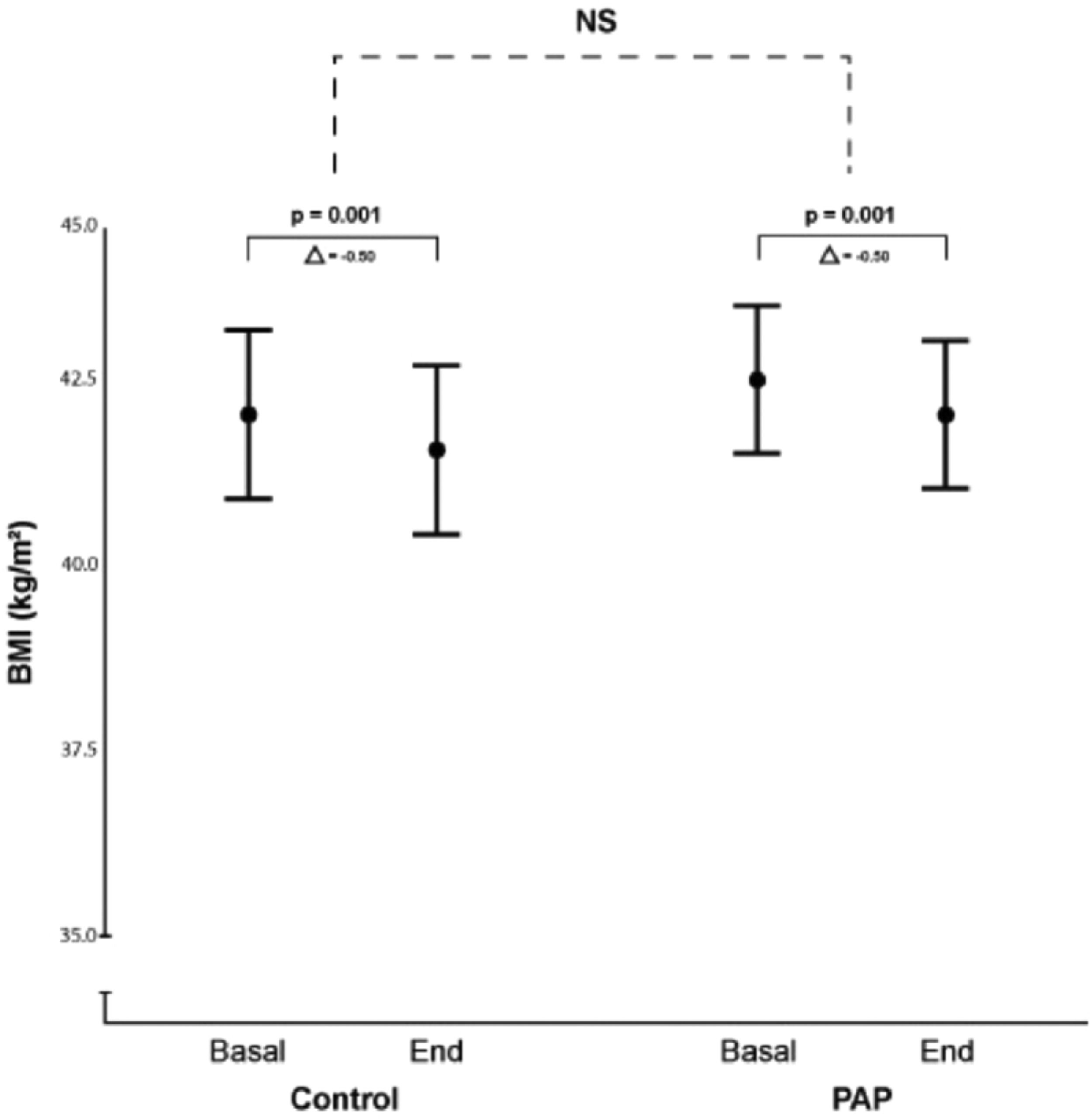
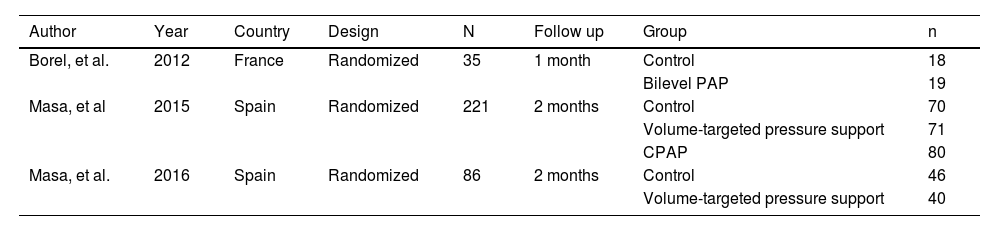
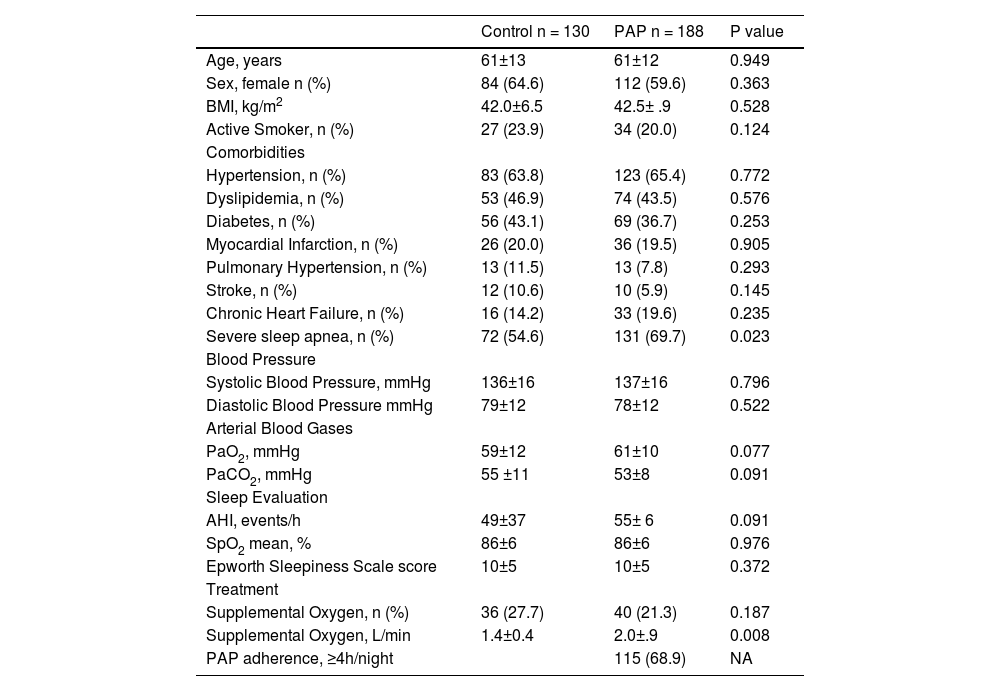
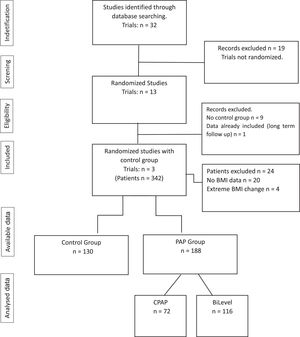
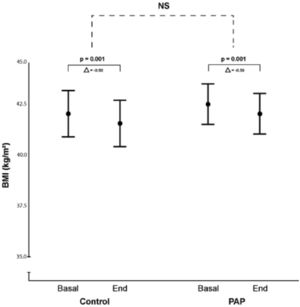
ResultsOur initial search retrieved 32 articles. However, 19 studies were excluded because they were not randomized controlled trials. Of the 13 randomized trials, 9 were also excluded because they did not have a non-PAP therapy as a control group12,20-28 and one study was the report of the long term follow up of a previously reported trial.10 Therefore, 3 randomized studies29-31 were included in the final analysis (Fig. 1: Flow chart of the study protocol). Quality assessment of randomized trials revealed that the risk of selection bias was low (supplementary file). Two studies used noninvasive ventilation, either in the form of bilevel PAP (follow up: 1 month),31 volume-targeted pressure support, vs control group (follow up: 2 months)30 and one study had three arms: CPAP or volume-targeted pressure support and a control group (follow up: 2 months).29 These 3 trials included 342 patients from France and Spain (Table 1). The final analysis consisted of 318 patients because 20 patients had missing BMI data and 4 patients had extreme BMI change (> 4 SDs). The majority of OHS patients were females (62%) and had at least mild OSA (AHI ≥5 events/h). The demographic and comorbidities of the population studied is summarized in Table 2. Patients randomized to PAP or control had similar characteristics, except for a higher percentage of severe OSA (AHI >30 events/h) in the PAP group as compared to controls (69.7% vs 57.1%, respectively; p=0.023). The mean PAP adherence of the pooled patients was 4.9±2.6 h/night, ranging from 4.5±2.829 to 5.6 ± 2.2 h/night.31 Adherence to CPAP and NIV was non different (5.1 ± 2.5 vs 4.7 ± 2.8 h/night, respectively; p=0.38). BMI decreased in both the control group (42.0 ± 6.5 vs 41.5 ± 6.7 kg/m2, p = 0.001) and the PAP group (42.5 ± 6.9 vs 42.0 ± 6.8 kg/m2, p = 0.001). The effect was not different intergroups (p=0.939) (Fig. 2). The average reduction in BMI in control and PAP groups were 0.50 ± 1.49 and 0.50 ±1.83, respectively). Adequate PAP adherence (>4 h/night) did not influence change in BMI (p = 0.118). Moreover, OSA severity, presence of heart failure, hypoxemia, hypercapnia and use of supplemental oxygen were also not associated with change in BMI. In contrast, higher age, higher diastolic blood pressure, lower oxygen saturation and higher AHI were independently associated measurements of BMI.
Characteristics of the selected randomized controlled trials studies.
PAP: positive airway pressure. CPAP: continuous positive airway pressure.
Demographic data of participants.
PAP: positive airway pressure. BMI: body mass index. AHI: apnea hypopnea index. SPO2: peripheral oxygen saturation. NA: not available; Mean±SD: standard deviation.
Body Mass Index (BMI) in control and PAP arms. Mean body mass index (BMI) and 95% CI at study entry and end of the study in the control group and positive airway pressure (PAP) therapy group. There was a significant BMI loss along the study that was independent of group allocation. Mean BMI and (95% CI)41 at baseline and end of study was 41.99 (40.86 – 43.13) and 41.51 (40.35 – 42.66), in the control group vs 42.46 (41.47 – 43.46) and 41.99 (41.02 – 42.96) Kg/m2 in the PAP group. NS (no significant).
This study was designed to elucidate the impact of treatment of OSA+OHS on BMI. Our main finding is that patients with OSA+OHS enrolled in randomized clinical trials lose weight. Therefore, PAP therapy in OHS does not lead to a higher BMI. Importantly, the impact of treatment of OSA+OHS on BMI in not influenced by the presence of absence of PAP, PAP compliance, OSA severity, presence of heart failure, level of hypercapnia/hypoxemia and use of supplemental oxygen.
Our study used BMI as the main endpoint because this was a consistent parameter across studies. We were not able to retrieve weight data from one study. However, considering a mean height of 1.62 m from the available data, the mean weight loss was in the range of 1.25 kg during a period ranging from 1 to 2 months of OSA+OHS treatment with or without PAP. This effect is in contrast with the average weight gain of 0.42 kg in eucapnic patients with OSA treated with CPAP during a period ranging from 1 to 48 months.14 In order to evaluate the independent effect of PAP on weight, we only included randomized trials that had a control group without PAP. However, our results are in line with the observation that the treatment of OHS with PAP is associated with weight loss, ranging from 4 to 6.7 kg, in the majority of the studies (periods ranging from 3 months to 3 years) that did not have a control group without PAP.10,12,25,28,32 Only one study found no significant changes in BMI among patients treated with 3 modalities of PAP modalities (CPAP, Bilevel and AVAPS).29 The use of GEE allowed us to control the effects of weight change for several variables such as PAP adherence, OSA severity, presence of heart failure, level of hypercapnia/hypoxemia, and supplemental oxygen. In all trials evaluated, participants were instructed to change eating habits with diet, increase overall daily physical activity, and to improve their sleep hygiene and habits. These factors can therefore explain why both PAP and control groups lost weight along the study period. This line of interpretation is in agreement with the Hawthorne effect that describes behavior changes or improvement even in the control group, due to the fact that the patient was enrolled in a research study.33 Although this is a tempting hypothesis to explain our results, weight loss is difficult to implement by lifestyle modification and is frequently unsuccessful amongst obese patients.34 It should be noted that the three included studies had rather a short duration of follow up (1 to 2 months). However, in a longer study of patients with OHS treated with PAP weight loss was maintained during at least 3 years.10 Finally, instructions on lifestyle modifications are also routinely given to obese patients with eucapnic OSA enrolled in randomized clinical trials, and therefore would not explain why patients with OSA gain weight when treated with CPAP compared to the control group which also received instructions on lifestyle modifications.
This study was not designed to explain the mechanisms associated with BMI changes in patients with OSA+OHS. Several mechanisms, including changes in overnight metabolism, basal metabolic rate, daytime physical activity and caloric intake have been considered in patients with OSA only treated with CPAP. In contrast to the hypothesis of weight change related to metabolism, one recent randomized study showed that weight gain occurs rapidly, after only one week of CPAP, raising question of whether such rapid shifts in weight can be attributed to metabolic changes. Furthermore, no changes in basal metabolic rate were noted.15 One randomized trial showed that fluid accumulation can lead to weight gain with CPAP therapy after just over one week of therapy.15 Fluid accumulation is a common effect of positive pressure ventilation in other settings, such as invasive mechanical ventilation.35 Other mechanisms could potentially explain the effect of PAP therapy on fluid balance in patients with OHS+OSA. The majority of patients in both control and PAP arms were hypoxemic at baseline (Table 2). Supplemental oxygen ameliorates the hypoxic vasoconstriction in the pulmonary circulation, unloads the right ventricle and the cardiovascular system and may reduce fluid accumulation.17,36 We therefore believe that changes in fluid balance is a unifying mechanism that may explain weight changes in patients with OHS+OSA treated with and without PAP. In fact, the percentage of patients with leg edema decreased similarly with weight loss at 3 years in OHS patients treated with PAP.10 However, we acknowledge that such hypothesis must be tested in future studies.
Our study has some limitations. The number of trials included in the analysis was limited to only three studies from Spain and France. However, the randomized trials without a control arm without PAP from Australia and England pointed in the same direction, and there is no rationale to suppose that our results are not applicable to other countries. Despite the relatively low number of studies that had a proper control arm without PAP our analysis was based on individual level data of 318 patients. Moreover, we only included randomized trials that had a control group without PAP treatment, allowing us to discriminate the effects of OHS treatment with and without PAP. Due to limitations in the data set we were not able to control for the use of diuretic medications. We were not able to evaluate body composition before and after the treatment, making it difficult to infer mechanisms that can explain our findings. Future randomized clinical trials with control and PAP group are needed to understand the effect of OHS treatment on fluid and body composition with tools such as bioelectrical impedance. The main result of our study was the observation that in contrast to weight gain associated with the treatment of OSA with CPAP, the treatment of patients with OSA+OHS is associated with weight loss. However, the changes in BMI were relatively small and were limited to a period of 2 months. Therefore, other interventions targeting weight loss in this population must be considered.37
In conclusion, patients with OSA+OHS enrolled in clinical trials experienced a reduction in BMI. In contrast to OSA, PAP therapy does not lead to weight gain. Although the effect was not associated with PAP, since patients without PAP also had BMI reduction, this observation is important because treatment-associated weight gain would raise concerns among patients that are already obese at baseline and in whom obesity plays a central role in the genesis of OHS. We speculate that this effect may be mediated by changes in fluid composition, but future studies are necessary to elucidate this hypothesis.
Funding sourcesResearch Support Foundation of the State of São Paulo (FAPESP).
Author contributionsRafaela G S Andrade: Study design, Data collection, analysis and manuscript preparation;
Juan F Masa: study design, analysis and manuscript preparation;
Jean-Christian Borel: study design and manuscript preparation;
Luciano F. Drager: study design, analysis and manuscript preparation;
Pedro R Genta: Study design, analysis and manuscript preparation;
Babak Mokhlesi: Study design and manuscript preparation;
Geraldo Lorenzi-Filho: Study design, analysis and manuscript preparation.