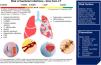
Lung transplantation can improve the survival of patients with severe chronic pulmonary disorders. However, the short- and long-term risk of infections can increase morbidity and mortality rates.
A non-systematic review was performed to provide the most updated information on pathogen, host, and environment-related factors associated with the occurrence of bacterial, fungal, and viral infections as well as the most appropriate therapeutic options.
Bacterial infections account for about 50% of all infectious diseases in lung transplanted patients, while viruses represent the second cause of infection accounting for one third of all infections.
Almost 10% of patients develop invasive fungal infections during the first year after lung transplant. Pre-transplantation comorbidities, disruption of physical barriers during the surgery, and exposure to nosocomial pathogens during the hospital stay are directly associated with the occurrence of life-threatening infections.
Empiric antimicrobial treatment after the assessment of individual risk factors, local epidemiology of drug-resistant pathogens and possible drug-drug interactions can improve the clinical outcomes.
The rising prevalence of some severe chronic medical conditions has increased the demand for organ transplantations worldwide. Lung transplantation (LT) has saved the lives of patients suffering from severe pulmonary diseases. However, immunodeficiency following the exposure to immunosuppressive drugs, prescribed to reduce the probability of rejection, increases the risk of acquiring life-threatening infections. Host-related biological agents and environmental factors can affect the infectious risk.
To better define the risk factors and the management of pulmonary infections in lung transplant recipients (LTRs) we performed a narrative review.
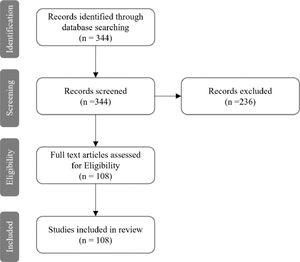
MethodsA narrative review was carried out to retrieve the scientific evidence on bacterial, viral, and fungal infections occurring in lung transplant recipients. The search engine Pubmed was used to select peer-reviewed articles published from 1/Jan/2010 to 31/Dec/2021. The References of the selected manuscripts were carefully assessed to collect articles not included in the primary search. The following key-words were used: “lung transplantation”, “infection”, “viral infection”, “bacterial infection”, and “fungal infection”. A total of 344 records were found. Based on titles, abstracts, and the content of the full texts, a total of 108 studies were deemed suitable. Twenty-three (21.3%) were focused on viral infections and lung transplantation, fifty-nine (54.6%) on bacterial infections, twenty (18.5%) on fungal infections, and six (5.6%) on parasitic infections (Fig. 1).
Bacterial infectionsBacterial infection in LT recipientsBacterial infections are the most frequent infectious complications following LT, accounting for ∼50% of all incident infectious diseases. Pneumonia represents the most frequent bacterial infectious disease after LT.1
The host predisposing factors and LT predisposing factorsPre-transplantation comorbidities or medical conditions of the LTR should be carefully considered when the risk of post-LT bacterial infections is computed: patients with cystic fibrosis (CF), diabetes mellitus, obesity or malnutrition, viral hepatitis, human immunodeficiency virus (HIV) infection, latent tuberculosis infection (LTBI), colonization by multi-drug-resistant (MDR) bacteria, chronic renal failure, and the need for mechanical ventilation are all risk factors for post-transplant bacterial infections.2
Moreover, immediately after surgery, LTRs are affected by a complete disruption or partial loss of physical barriers and are at risk of the following medical conditions: aspiration pneumonia, central line-associated bloodstream infection (CLABSI), ureteral catheter-associated infection, Clostridioides difficile colitis, and sepsis.2
During the early post-LT period (a few days to 1 month later), nosocomial microorganisms account for the majority of infections; LTRs with CF are more likely to develop colonization and recipient- or donor-harbored infection infection by Pseudomonas aeruginosa, non-tuberculous mycobacteria (NTM), and Burkholderia cepacia complex.2,3
LT procedures associated with a transient disruption of the bronchial circulation may cause the loss of the epithelial integrity, as well as ciliary function and mucus production, increasing the risk of infection.2,4
Denervation of the allograft may suppress the cough reflex, promoting bacterial colonization and bronchial inflammation.5 Impaired lymphatic drainage of the allograft may result in stasis and oedema of the bronchial tissues, slowing normal healing and promoting superinfection.5
In the case of necrosis of the bronchial anastomosis, bacterial colonization can increase due to a reduced clearance of secretions.5
Immunosuppression, as well as the type of immune-suppressant agents, to prevent reject of the allograft may lead to prolonged T- and B-cell dysfunction, and/or macrophage and cytokine dysregulation (mainly caused by corticosteroids), exposing LTRs to the risk of bacterial pathogens (e.g., Nocardia spp., Mycobacterium tuberculosis).
Epidemiology of bacterial infection post LTBacterial infections, mainly located in the respiratory tract, are more frequent in LTRs than in other solid organ transplant (SOT) recipients: 35%–66% of the infections in LTRs are caused by bacteria, with 50–85% of recipients showing a bacterial complication after transplantation.2,6,7
LTRs may experience >1 bacterial infection with >1 bacterial pathogen.5,8 The risk is highest during the first 3 weeks after LT, with P. aeruginosa, Enterobacterales, Staphylococcus aureus, and Enterococcus spp. being the most incident pathogens.9,10
However, infections caused by Acinetobacter spp., Stenotrophomonas maltophilia, and other Gram-negatives are often diagnosed.
The prescription of antibiotic prophylaxis has postponed the occurrence of most cases of bacterial pneumonia to the intermediate and late postoperative period,5 including tuberculosis (TB) if LTBI is not treated after the LTBI diagnosis.11 NTM disease rate, caused mainly by Mycobacterium avium complex, followed by Mycobacterium abscessus, is higher among LTR than in the other SOT recipients.12,13
Clostridiodes difficile infection, with a highest incidence during the first month after LT, is associated with the use of antibiotics, proton-pump inhibitors (PPIs), and steroids in the immediate pre-LT period.14–16
Screening and antibiotic prophylaxis in LT recipientsPre-LTScreening for infections of transplant candidates and living donors is essential to increase the probability of LT success. Vaccinations can help prevent bacterial infections (e.g. Streptococcus pneumoniae).
Medical history should be carefully evaluated: previous infections and their therapy, comorbidities, lifestyle, travel history, allergy, contact with TB patients, exposure to antibiotics, animal, and environmental pathogens can help tailor antibiotic prophylaxis.17 Colonization by MDR Gram negative and positive bacteria should be investigated before transplantation; therefore, rectal (K. pneumoniae producing extended-spectrum β-lactamase (ESBL), metallo-β-lactamase (MBL), carbapenem-resistant(KPC)), E. coli (ESBL, KPC, MBL), P. aeruginosa (MDR), Acinetobacter spp. (MDR), Enterobacter spp. (MDR), S. maltophilia, Enterococcus spp. (vancomycin resistant Enterococci VRE)) and nasal swabs (methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), vancomycin intermediate Staphylococcus aureus (VISA)), as well as respiratory cultures, are needed to decrease the risk of a difficult-to-treat infection. Unfortunately, colonization or previous or active infection caused by Burkholderia cepacia complex and M. abscessus are associated with poor post-transplant outcome: their occurrence could constitute a contraindication for transplantation.18–20 Serological testing for some sexually transmitted diseases (e.g., syphilis) should be performed in both transplant candidates and living donors.19 Donors suspected of having TB should not be considered, whereas LTRs should be screened for LTBI with the interferon-gamma release assay (IGRA) and, if tested positive, a chest-X-ray and molecular, microscopic, and culture and drug susceptibility testing on sputum samples should be performed to exclude active TB.19 If TB or LTBI is diagnosed, the patient should be immediately treated.21
Surgical site infections (soft tissue infection and/or mediastinitis and/or airway anastomosis infection) may occur in ∼20% of LTRs.22,23 If the pre-LT screening is negative, cefazolin can be prescribed for prophylaxis and should be administered 1 h before incision23; in the case of an allergy to penicillin, levofloxacin could be an alternative option.23 On the other hand, if MDR pathogens are detected during screening, the choice of antibiotic should depend on the drug susceptibility profile and on the risk factors for surgical site infection (e.g., if MRSA is detected, linezolid or vancomycin can be coupled with an antibiotic against Gram negatives).23
The optimal duration of prophylaxis is yet to be elucidated; however, American Society of Transplantation guidelines recommend their prescription for 48–72 h.23
Trimethoprim-sulfamethoxazole is administered to reduce the incidence of Pneumocystis jirovecii, Nocardia spp., and Listeria monocytogenes infections.24
Post-LTBlood stream infections (BSI) caused by Staphylococcus aureus and Pseudomonas aeruginosa may result in early post‐transplant sepsis or mycotic aneurysm at the site of allograft vascular anastomoses; therefore, blood cultures should be performed and empiric antibiotics should be prescribed.25
Pneumonia can be diagnosed in the late post-LT period and it can be health care-associated (potentially caused by MDR pathogens) or community-acquired (CAP).26
During a median follow-up of 1.3 years after LT, 6.4% developed invasive infection by S. pneumoniae, highlighting the importance of routine pneumococcal vaccination.26
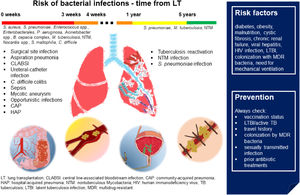
Fig. 2 sums up epidemiology, risk factors, and prevention of bacterial infections in patients with LT.
Bacterial infections in LT. In the first month after LT, in addition to community- and hospital-acquired pneumonia, patients are at higher risk of surgical site infection, central line-associated bloodstream infection, ureteral-catheter infection, sepsis, mycotic aneurysms. Immunosuppression due to transplantation and the antibiotic prophylaxis increases the risk of opportunistic infections and C. difficile colitis. In the late post-LT period, tuberculosis reactivation, nontuberculous Mycobacteria and S. pneumoniae infections are the most frequent bacterial infections. Risk factors and suggestions on how to prevent bacterial infections are shown in the blue box. (Source: authors elaboration).
Empirical antibiotic therapy should be tailored on patients’ characteristics: previous allergy to antibiotics, potential drug–drug interactions, bacterial colonization (MDR pathogens) or previous infections, and local epidemiology (hospital where the LT is performed and where the donor lives).5,27 A diagnostic work-up with blood cultures and, in the case of respiratory symptoms or signs, bronchoscopy, bronchoalveolar lavage (BAL), and transbronchial lung biopsy should be performed before any antibiotics are prescribed. Following the collection of samples for cultures, broad-spectrum antibiotics can be administered and, when the bacteriological results are known, specific antibiotics can replace the initial therapy.5,27,28 Therapeutic drug monitoring (TDM) should be performed to assess the blood concentration of antibiotics and to reduce the risk of side effects.
Pseudomonas sppLTRs with a history of P. aeruginosa colonization are usually treated with two effective drugs for 2 to 3 weeks after surgery to reduce the risk of pneumonia and allograft colonization.29,30
Systemic colistin with systemic and/or aerosol aminoglycosides (AG) should be cautiously prescribed due to the risk of cumulative nephrotoxicity (and the dosage of AG should be carefully chosen considering renal function) when combined with calcineurin inhibitors for immunosuppression; if drug-susceptibility testing (DST) shows cephalosporin susceptibility, ceftazidime (2g IV q8h) or cefepime (2 g IV q8h) associated with aerosol or intravenous AG can be administered. Fluoroquinolones and piperacillin/tazobactam (4.5g IV q6h) are effective alternative options. If ESBL-producing P. aeruginosa is diagnosed, carbapenems (meropenem 2g IV q8h or imipenem/cilastatin 1g IV q6h) or ceftolozane/tazobactam (3g IV q8h) can be prescribed. In the case of a serin-carbapenames-producing P. aeruginosa, ceftazidime/avibactam (2.5g IV q8h) or meropenem-vaborbactam (4g IV q8h) should be preferred in combination with aerosol and intravenous colistin, systemic fosfomycin, or aerosol and intravenous AG.5,29,31 If metallo-carbapenemase is detected, ceftazidime/avibactam (2.5g IV q8h) in association with aztreonam (2g IV q6h) can be administered. Monotherapy with aerosol and intravenous colistin could be another option.
Future options may be aerosol fluoroquinolones, imipenem/relebactam, cefiderocol, and murepavadin.31,32
Anti-pseudomonal agents can be associated with the following issues: drug-drug interactions (DDIs), allergy, and impairment of renal function and drug volume distribution (e.g., obese patients, septic shock).33
Acinetobacter sppEmpiric antibiotic treatment aimed to treat Acinetobacter spp. should rely on local epidemiology: meropenem (2 g IV prolonged infusion over 3 h every 8 h) can be administered in critically ill patients. After DST results, de-escalation to cefepime (2g IV q8h) should be recommended. However, if pan-resistant Acinetobacter is detected, systemic (with loading dose) and aerosol colistin, with or without cefiderocol or meropenem in continuous infusion, can be prescribed.31,32,34
Staphylococcus aureusStaphylococcus aureus pneumonia or BSI in a LTR should be immediately treated to avoid necrotizing pneumonia.35
Active S. aureus screening with nasal swabs before LT is highly recommended to avoid post-LT S. aureus infection: pre-transplant S. aureus colonization in CF patients undergoing LT increases the risk of post-transplant infection.36
If pre-LT screening did not show MRSA colonization, empiric treatment with cefazolin (2g IV q8h) or oxacillin (2g IV q4h), or linezolid (600 mg IV q12 h) (if penicillin or cefalosphorin allergies are reported) can be started.37–39 In case of MRSA colonization, ceftaroline (600 mg IV q8h) or linezolid (600 mg IV q12 h) should be preferred.
Enterococcus sppIn the case of an infection caused by ampicillin-susceptible Enterococcus spp., ampicillin- or amoxicillin-based combination therapy with ceftriaxone (in case of bacteremia, 2g q24h) or aerosol and systemic AG (pneumonia with or without bacteremia) can be administered.40,41 In case of VRE, linezolid (600 mg q12h) or tedizolid (200 mg q24h) should be preferred.40
TuberculosisThe culture-based diagnosis of tuberculosis should be supported by the assessment of the drug resistant patterns, if any.42,43 Pulmonary resection can be performed in difficult-to-treat cases.44 Ideally, screening for Mycobacterium tuberculosis infection in potential organ donors should be systematically implemented to reduce the risk of transmission.45
Burkholderia cepacia complexInfection caused by Burkholderia cepacia complex (BCC) can be life-threatening in CF patients and could constitute a contraindication for LT; however, if the infection is successfully treated prior to the surgical intervention, LT can be performed.46,47 BCC infection should be managed by reference centers (definition based on their long experience in the management of BCC in CF)47: meropenem, ceftazidime, piperacillin, and trimethoprim-sulfamethoxazole show activity against BCC.48,49 Combination of the above-mentioned drugs can increase the treatment success rate.48,49
Nocardia sppNocardia is a well-recognized pathogen in immunocompromised hosts. Pulmonary infections in LTRs can be associated with the involvement of other organs (such as CNS).50,51
Trimethoprim/sulfamethoxazole (160/800 mg per os q12h or 15 mg (based on trimethoprim)/Kg/day IV) in combination with meropenem (2g IV q8h) is the standard of care.52 Linezolid (600 mg IV q12 h) with meropenem (2g IV q8h) or imipenem/cilastatin (500 mg IV q6h) can represent an alternative. The duration of therapy depends on the clinical and microbiological response (up to nine months in some cases).51
Stenotrophomonas maltophiliaThe diagnosis of a colonization or infection caused by S. maltophilia is challenging; in cases of infection, trimethoprim/sulfamethoxazole (160/800 mg orally q12h or 15 mg (based on trimethoprim)/Kg/day IV) or levofloxacin (750 mg IV daily) or minocycline (200 mg IV loading dose, then 100 mg q12h) can be administered.53 Omadacycline, eravacycline, and cefiderocol are active in vitro, but no clinical data are available.54,55
Carbapenemase-producing Enterobacterales (CPE) colonization and infectionActive screening with rectal swab and BAL for MDR Gram-negative bacteria should be implemented.31,56
Empiric treatment with ceftazidime/avibactam (2.5g IV q8h), or meropenem-vaborbactam (4 g IV q8h), or imipenem/relebactam (1.25 g IV q6h), or ceftazidime/avibactam (2.5 g IV q8h) in association with aztreonam (2 g IV q6h), with or without aerosol and intravenous colistin, systemic fosfomycin, or aerosol and intravenous AG, can be prescribed depending on the local CPE epidemiology.31,57
Cefiderocol (2 g IV q8h) can be administered in case of pan-resistant isolates.31
Reasoned empiric treatment of bacterial, viral and fungal infections in LT is presented in Table 1.
Reasoned antimicrobial therapy for most common pathogens complicating lung transplantation.
Dosages refer to adult patients with normal renal function.
LT: lung transplantation; MDR: multidrug-resistant; PDR: pandrug-resistant; AST: antimicrobial susceptibility testing; MRSA: methicillin-resistant S. aureus; VRE: vancomycin-resistant Enterococcus; CRE: carbapenemase-producing Enterobacterales; IV: intravenous; PO: per os; CI continuous infusion; LD: loading dose; MIU: millions international units; AmB: amphotericin B; ABLC: amphotericin B lipid complex; TMP-SMX: Trimethoprim-sulfamethoxazole.
Viruses represent the second cause of infection in LTRs, accounting for one third of all infections.58 Their relevance increases with the immunosuppression peak, which can facilitate the reactivation of latent opportunistic infections.1 The risk decreases when immunosuppression reaches maintenance levels, although it could increase following the exposure to community-acquired respiratory viruses (CARVs),1 which can be easily diagnosed with molecular diagnostic techniques.59
Cytomegalovirus (CMV) is the most frequently-detected viral agent during the first month and from the second to the sixth month (75% and 80%, respectively); however, it could promote other infections, such as Epstein-Barr virus (EBV) related lymphoproliferative disorders, and acute and chronic rejection.60,61 CARVs account for about half of viral infections after 6 months.59,62
Host predisposing factors and LT predisposing factorsThe main predisposing factors for a viral infection are the significant anatomical and functional modifications caused by transplant surgery and the immunosuppression to prevent rejection. Being carrier of a viral infection (both donor and host) can be associated with a higher risk of disease although systematic screening could lower this risk.
The screening of recipients enables an assessment of the eligibility for LT and to plan vaccinations and other preventive interventions.
The highest risk of CMV infection (defined by the American Society of Transplantation as evidence of CMV replication regardless of symptoms) and disease (evidence of CMV infection with attributable symptoms) can be explained by the pulmonary lymphatic reservoir and the high level of immunosuppression.63 A discordant serological status “donor-positive (D+)/recipient-negative (R-)” can be dramatic for the risk of disease.64
EBV, which causes a latent infection in 90–95% of adults, can lead to severe and disseminated infection and to post-transplant lymphoproliferative disease when transmission occurs from a D+ to a R- (10% of LT).65,66 High-level immunosuppression may contribute by promoting EBV-infected B cell escape from T-cell mediated immune surveillance.67 Several studies highlighted the association between some induction agents (e.g., OKT3), higher EBV viremia, and risk of lymphoproliferative disorders.68
LTRs and their households should be counselled on their behaviors, including hand washing and droplet precautions, and be vaccinated against influenza annually. Reduction of immunosuppression, when possible, can help reduce the risk of severe disease.1
Less common viral infections caused by hepatitis E virus (HEV), West Nile virus (WNV), Human T-lymphotropic virus (HTLV)-1/2, rabies, Zika virus, and lymphocytic choriomeningitis virus (LCMV) should be evaluated taking into account some important factors, such as travel and residence history, past and current employment, lifestyle, and exposure to animals.69
Pre-LT candidate screeningLT candidates are routinely screened for the following agents: herpesviruses (HSV-1 and HSV-2, EBV, CMV, varicella zoster virus or VZV), viral hepatitis viruses (HAV, HBV, HCV), HIV, measles, mumps, and rubella (MMR).19
In cases of acute disease, transplantation is usually postponed.70
CMV-specific IgG test shows high sensitivity and specificity; in the case of negativity, a second test should be performed immediately before transplant; uncertain results should be considered positive and the patient at high risk.71
The incidental diagnosis of a chronic viral infection (e.g., HIV, HCV, HBV) can lead to a reassessment of LT feasibility and timing.72–76
Serology related to vaccine-preventable diseases (e.g., HAV and HBV, MMR, and VZV) is mandatory to plan an immunization program before LT, mainly for live attenuated vaccines (e.g., MMR and VZV vaccines).77
Pre-LT donor screeningDonor serological status for HIV, HBV, HCV, CMV, EBV, and WNV should be carefully evaluated when the lung explant is scheduled (and not >28 days before) and matched with the serostatus of the recipient.19,78
Nucleic acid amplification testing (NAT) is recommended routinely for HCV, HIV, and HBV (in case of highest risk).19,78,79
In deceased donors, NAT for HIV, HCV, and HBV is recommended and results, if not available before transplant, can be used to prescribe the recipient's treatment.78,79
HIV-1/2 screening with fourth-generation test (or alternatively, third-generation combined with NAT) is recommended.
HBV infection should be screened evaluating HBsAg, HBcAb IgM and IgG, whereas NAT is recommended in deceased donors and those deemed at highest risk.19,78 Transplant is contraindicated when the donor is HBsAg and/or HBcAb IgM positive and recipient is HbsAb negative; if the recipient is HBsAb positive, a life-saving transplant associated with pre-emptive therapy should be considered.19 In cases of transplant from donors with only HBcAb IgG, the serological recipient profile should be carefully considered and different preventive approaches should be adopted: prophylaxis with HBIG and antivirals in the case of positive HBsAb or positive HBsAb and negative HBcAb, no prophylaxis in case of positive HBsAb and HBcAb.19
Three donor types can be identified after HCV screening: uninfected donors (HCVAb/NAT negative); donors with cleared infection (HCVAb positive/NAT negative), considered similar to uninfected donors80,81; actively infected donors (HCVAb/NAT positive), which should be taken into consideration when highly active antiviral therapies are available.82–84
Assessment of CMV and EBV serostatus (CMV IgG and viral-capsid antigen VCA-IgG, respectively) of donor and recipient is key to define pre-transplant prevention planning, as well as the risk of post-transplant EBV-associated lymphoproliferative diseases (PTLD).71,85 The “D+/R-” status is at higher risk of severe infection.19
In t case of CMV-specific IgG negativity at the first evaluation, a second test should be performed immediately before transplant; uncertain results should be considered as positive and the combination “donor–recipient” at the highest risk.71
Focused screening of less incident viruses should be addressed based on clinical and epidemiological data.19,69,86
Post-LT monitoring and preventionThe risk of life-threatening post-LT disease and the opportunity for prevention and/or early treatment justify post-LT systematic monitoring for CMV and EBV.
CytomegalovirusMonitoring of CMV DNAemia by quantitative nucleic acid amplification testing (qNAT), in LTRs is the gold standard for a prompt diagnosis of CMV infection and disease.71,87 Whole blood positivity is helpful for an early assessment of the infection, whereas plasmatic persistence of DNAemia is a predictor of treatment failure.88 Both whole blood and plasma can be used but the same specimen should be used during the monitoring process.71 Antigenemia testing (pp65) in peripheral white blood cells is an alternative option, but it is labor-intensive and less accurate.89
CMV prevention strategies are based on two approaches: “universal prophylaxis” and “preemptive therapy”.
Universal prophylaxis depends on the immediate administration (within 10 days after LT) of a preventive dose of antivirals in at-risk LTRs for a definite period of time. Based on the high rates of CMV infection (R negative) and reactivation (R positive) this approach is strongly recommended.71
Intravenous ganciclovir 5 mg/kg once daily is the drug of choice and can be switched to oral valganciclovir 900 mg once daily when oral administration is feasible; both drugs need dose adjustment in cases of renal disfunction.71
A therapy duration of 6-12 months is recommended: 12 months in D+/R-, whereas at least 6 months in D+/R+ and D-/R+.71,90 Prophylaxis is not routinely recommended in D-/R-; thus, prophylaxis of herpes virus and varicella (acyclovir, valacyclovir or famciclovir) should be evaluated.71
Preemptive therapy is based on the regular monitoring of CMV replication (e.g., weekly) for 3–4 months after LT, in order to promptly prescribe treatment to prevent the disease. Longer intervals of screening are associated with an increased incidence of disease and shorter survival.71,91 Once a critical threshold (not universally established) is achieved, valganciclovir should be started. Currently, preemptive therapy is not recommended in LT because of the high risk of CMV disease and the limits of screening standardization.71,92
The occurrence of post-prophylaxis (or late-onset) CMV disease, possibly associated with a reduced cell-mediated immunity, is frequently observed in cases of LT, high-dose immunosuppression, universal prophylaxis ≤ 6 months and D+/R- serostatus.71,90,93 Weekly surveillance after prophylaxis for 8–12 weeks should be considered.71
Epstein-Barr virusEBV seronegative recipients are at high risk of primary infection and PTLD (highest incidence within 1 year from transplant)94; thus, EBV-DNA monitoring and early treatment should be considered. Prophylaxis or pre-emptive strategies with acyclovir or ganciclovir are supported by poor data.95 Blood EBV-DNA should be monitored monthly for 6 months, followed by an assessment every 3 months until 12 months post LT.86
Other herpevirusesThe high prevalence of HSV-1/2 and VZV latent infection both in donors and recipients and the availability of effective prophylaxes and VZV vaccination make those infections of minor concern. Furthermore, anti-CMV agents can prevent other Herpesvirus infections. In the case of a CMV pre-emptive strategy and seronegative recipients, prophylaxis with acyclovir 400–800 mg po every 12 h (alternatively, valacyclovir 500 mg po every 8–12 h or famciclovir 250 mg po every 12 h) is recommended during the first month after transplant and during periods of intensified immunosuppression.96 In cases of exposure to a patient with varicella and a recipient seronegative status, prophylaxis with acyclovir or valacyclovir is recommended and Varicella-zoster immunoglobulins (VZIG) (125 units/10 kg IM, minimum dose of 125 units, maximum of 625 units) should be administered within 96 h (up to 10 days).97–99
Pre-LT screening for HHV8 (also known as Kaposi's sarcoma-associated herpesvirus -KSHV) is recommended100,101: in cases of HHV8 D+/R- transplant close monitoring of HHV8-DNA in the blood is recommended.86
Community-acquired respiratory virusesCARVs includes human rhinovirus, influenza, parainfluenza, adenovirus, respiratory syncytial virus (RSV), and coronavirus (CoVs), which can cause lower respiratory tract infections, acute and chronic rejection, and death.102,103
Prevention is based on hand hygiene and precautions against transmission of droplets; accurate diagnostics (PCR-based testing on nasopharyngeal swab, bronchial aspirate, or BAL) are key for early diagnosis.
Currently, immunization is available against influenza, RSV and SARS-CoV-2.
Pre-exposure prophylaxis should be considered during the influenza season or when vaccination is contraindicated or expected to confer low protection (e.g., high-level immunosuppression).104 Post-exposure prophylaxis with oral oseltamivir or inhaled zanamivir (75 mg po and 10 mg respectively, once daily, respectively) should be administered to unvaccinated LTRs within 48 h of contact and for 5–10 days afterwards.1 Oseltamivir is recommended over zanamivir in the U.S. for the treatment of influenza. In the countries where it is available, zanamivir represents an alternative to oseltamivir and it is recommended for the treatment of uncomplicated influenza A and/or B in patients aged 7 years and older with symptoms for no more than 2 days. Of note, Zanamivir should not be administered in intubated patients. In LTRs under prophylaxis who develop flu-related symptoms, prompt testing and initiation of therapy treatment is recommended.104
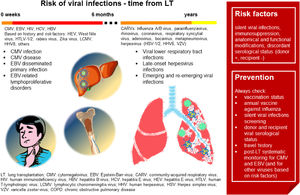
Fig. 3 summarizes the main characteristics of viral infections in LT.
Viral infections in LT. In the first six months after LT, CMV infection and disease is the most frequent viral infection, followed by EBV disseminated primary infection and EBV-related lymphoproliferative disorders. Based on individual risk factors, viral hepatitis, HIV, West Nile virus, HTLV-/2, rabies virus, Zika virus, LCMV and HHV8 infections should be investigated. In the late post-LT period, community-acquired respiratory viruses represent the main risk. Risk factors and suggestions on how to prevent viral infections are shown in the red box. (Source: authors elaboration).
Oral valganciclovir (900 mg every 12 h) and intravenous ganciclovir (0.5 mg/kg every 12 h) are the first-line drugs in cases of mild-to-moderate and severe-to-life-threatening disease, respectively. Ganciclovir is recommended in cases of gastrointestinal CMV disease or in cases of reduced bioavailability.71
Blood counts and renal parameters should be strictly monitored to assess their toxicity.
DNAemia should be monitored weekly; treatment should last ≥2 weeks up to clinical recovery and DNAemia eradication (a single negative determination with a highly sensitive test, or two consecutive negative results with a less sensitive test).71,105
In the case of treatment failure (persistent high DNAemia or its increase during treatment), the most prevalent resistance mutations (UL97 and UL54) should be evaluated in order to perform a therapeutic switch to second-line antivirals (foscarnet, cidofovir).106,107
Secondary prophylaxis in not associated with fewer relapses and is not routinely recommended.108,109
Epstein-Barr virusA significant increase in EBV-DNA (e.g., >103 IU/mL) should be rapidly investigated and may require a prompt reduction of the immunosuppression level, as well as the prescription of other therapeutic approaches (e.g., rituximab).86
Other herpevirusesTreatment of HSV1-1/2 and VZV infection depends on their clinical manifestations97,110 (Table 1).
Community-acquired respiratory viruses (CARVs)Treatment of influenza should be started ideally within 48 h following the onset of symptoms, but may be beneficial up to 5 days afterward; 10 or more days should be considered according to severity and immunosuppression and dose adjustment based on renal function is recommended104,110 (Table 1). Treatment of RSV and Parainfluenza virus are reported in Table 1.111-113
LT and SARS-CoV-2 infection (COVID-19)Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is an emerging pathogen isolated for the first time in December 2019 in Wuhan, China.114 Due to its airborne transmission and high contagiousness, it resulted in a pandemic, officially declared by WHO in March 2020.115 The International Society of Heart and Lung Transplantation (ISHLT) promptly released guidelines that have recently been updated.116
Host predisposing factors and LT predisposing factorsSeveral retrospective cohort studies reported controversial results regarding the outcome of LT recipients affected by COVID-19.117–122 LTRs should be cautiously considered at high risk of developing COVID-19.116,123
The risk of disease decreases when medical facility visits are reduced and social interactions minimized.116
Pre-LT candidate screeningThe risk of exposure should be evaluated in LT candidates and a PCR test for SARS-CoV-2 should be performed. Candidates may be considered suitable for LT if:
- •
Asymptomatic with a negative PCR test.
- •
Symptomatic with a negative PCR test and an alternative diagnosis which does not contraindicate LT.
- •
Exposed but asymptomatic >7 days after exposure with two negative PCR tests performed 24–48 h apart, and at high risk of death without organ transplantation.
- •
Previously symptomatic, following clinical resolution and at least 14 days from the onset of symptoms, with two negative PCR tests performed 24–48 h apart, and without COVID-19 related organ damage, at high risk of death without organ transplantation.
- •
Asymptomatic with positive PCR test, at least 14 days after diagnosis with two negative PCR tests at least 24–48 h apart.
LT donors should be screened to assess the exposure risk and should undergo a PCR test for SARS-CoV-2 <72 h before organ explant.116 Their organ may be considered suitable for LT if:
- •
Asymptomatic with a negative PCR test.
- •
Symptomatic with a negative PCR test and an alternative diagnosis which does not contraindicate organ donation.
- •
Exposed but asymptomatic >7 days after exposure with two negative PCR tests performed 24–48 h apart (at least one a respiratory sample of the lower airways), without COVID-19 pulmonary involvement and negative CT, with a recipient candidate showing to be at high risk of death without LT.
- •
Previously symptomatic, following clinical resolution and at least 28 days from the onset of symptoms, with two negative PCR tests performed 24–48 h apart (including a respiratory sample of the lower airways), negative chest CT, and no other COVID-19 related organ damage.
LTRs should be monitored in accordance with international guidelines and local policies.
Antiviral therapies and open issues on COVID-19 and LTTo date, no consensus guidelines on treatment of COVID-19 in SOT recipients have been issued. DDIs and cardiac and pulmonary diseases represent the major concerns. Prescription of protease inhibitors (lopinavir/ritonavir, darunavir/ritonavir and darunavir/cobicistat) and chloroquine/hydroxychloroquine has been discouraged due to the lack of evidence and the high risk of DDIs and adverse events (e.g., QTc prolongation).124–128 Preliminary data on remdesivir suggest a low risk of significant DDIs.116
The inclusion of SOT recipients in COVID-19 clinical trials is urgently needed.
Parasitic infectionsWhile parasitic infections are rarely reported in LTR, careful assessment of pre-transplant risk factors of the LTR and the donor (epidemiology, possible exposure, travel history, previous immunosuppressive treatments) needs to be carried out.129–131
Trypanosoma cruzi and Strongyloides spp. may cause disseminated disease in immunocompromised patients, with high morbidity and mortality.132–134
Fungal infectionsEpidemiology of fungal infections post LTAccording to the Transplant Associated Infection Surveillance Network (TRANSNET), 8.6% of LTRs develop invasive fungal infections during the first year after LT,135 although the incidence can vary based on various relevant clinical and epidemiological drivers (e.g., patient population, immunosuppressive drugs, antifungals and antibiotic prophylaxis.).136 Fungal pneumonia contributes to 35% of pneumonia-related deaths during the first year after LT.137
The most frequent fungal pathogens are Aspergillus spp. (44%; mainly Aspergillus fumigatus), Candida spp. (23%; mainly Candida albicans), Scedosporium spp. and Fusarium spp. (20%).136,137 The incidence of Pneumocystis jirovecii pneumonia (PJP) has decreased owing to effective prophylaxis, whereas other molds (e.g., Criptococcus neoformans, Mucor, Rhyzopus) account for a reduced proportion of cases.
The host predisposing factors and LT predisposing factorsSeveral host and environmental factors can increase the risk of invasive fungal infection (IFI) following LT. In particular, anatomic and physiologic impairment and environmental exposure (e.g., farming and construction, sandblasting, air conditioning filters, flooded sites) increase the risk of IFI.
Airway ischemia, neutropenia, hypogammaglobulinemia, T-cell depletion and an over-immunosuppressed state, the need for bronchial stents, co-infection with CMV disease, and previous colonization with Aspergillus are all risk factors for IFI.138 Patients with cystic fibrosis are often colonized with fungi, while receiving a single-lung transplant poses a higher risk of invasive fungal infection compared with double-lung transplant recipients.
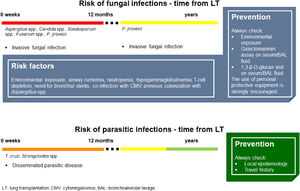
Fig. 4 summarizes the main characteristics of fungal and parasitic infections complicating LT.
Fungal and parasitic infections in LT. Invasive fungal infections in LTRs mainly occur within 12 months after LT. Aspergillus spp. and Candida spp. are the most frequent fungi responsible for infections in this setting. Asking for environmental and occupational exposure is fundamental to raise suspicion on high-risk patients. Based on individual risk factors, galactomannan and 1,3-β-D-glucan should be investigated. Parasitic infections in LTRs rarely occur, but the local epidemiology and personal travel history should always be kept in mind. (Source: authors elaboration).
Correct identification of patients at increased risk of fungal infection is key to IFI prevention.
LTRs have a significant risk of IFI caused mainly by Candida spp. and Aspergillus spp. A definitive diagnosis of IFI (such as invasive aspergillosis, and invasive candidiasis) requires histological evidence for infection or positive culture.139 Nevertheless, the use of nonculture diagnostic tests facilitates the detection of IFI.29
According to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID),139 mannan and anti-mannan detection enable the identification of Candida spp. in serum samples, whereas serum 1,3-β-D-glucan testing is often used as a first-stage screening test, as it is present in large amounts of fungal species,139,140 The application of galactomannan assay to BAL fluid coupled with serum galactomannan and serum 1,3-β-D-glucan expedites the diagnosis of invasive pulmonary aspergillosis in organ transplant recipients,141
Immunofluorescence microscopy and real-time PCR techniques on BAL are the recommended methods for PJP diagnosis; serum 1,3-β-D-glucan has a high negative predictive value in HIV patients, while increased levels can only support diagnosis.142
Prophylaxis in LTRsMinimization of environmental exposures and the use of personal protective equipment can reduce the risk of colonization and infection.138
A preemptive therapy can be administered. Nevertheless, no consensus on the antifungal agent, route of administration, and duration of prophylaxis has been reached.136
Inhaled amphotericin B is commonly used, as it is active against the most prevalent fungi. Optimal dosage, formulation, and duration are not standardized.139
Triazoles are commonly used for antifungal prophylaxis and voriconazole is the most frequently administered,29,138,139 followed by itraconazole143 and posaconazole.144 In particular, posaconazole appears effective in the management of IFI after LT, and well-tolerated. It could be considered if the use of traditional antifungal treatments is limited by drug resistance, drug interactions, and toxicities.144
Isavuconazole has been identified as non-inferior to voriconazole in invasive mold infections caused by Aspergillus and other filamentous fungi.145,146 Isavuconazole appears to be active against all Candida species and the most common Aspergillus species.147 Nevertheless, an increased incidence of IFI in neutropenic hematologic malignancy patients and hematopoietic cell transplant recipients during isavuconazole prophylaxis has been observed.148 This suggests the need for additional studies to determine the role of isavuconazole as prophylaxis in such patients.
Echinocandins show activity against Candida spp., Aspergillus spp., and other molds. However, they are not active against Cryptococcus spp., Histoplasma capsulatum, Coccidioides immitis.
LT is burdened with the higher risk of PJP among SOT recipients. Prophylaxis is recommended for a minimum of 6–12 months (in the case of standard risk) to lifelong (reserved for high risk).149 Trimethoprim‐sulfamethoxazole (TMP‐SMX) 160 mg/800 mg orally daily or three times weekly is the drug of choice, while dapsone (50–100 mg po daily), atovaquone (1500 mg po daily) and aerosolized pentamidine (300 mg q 3‐4 wk) are alternative agents in the case of TMP-SMX intolerance.150 The emerging occurrence of late onset PJP after 12-month prophylaxis supports the usefulness of an as-long-as-possible prophylaxis in LTRs.151
TreatmentAspergillusVoriconazole is the drug of choice for the treatment of invasive aspergillosis.29,139,149,152 Weight-based dosing is recommended to achieve optimal therapeutic ranges, with incremental increases and monitoring (i.e., 50% increase in daily dose) for those with trough levels <1 µg/mL. Target levels should range from >1 to <5.5 µg/mL (Table 1). Voriconazole can increase the levels of some immunosuppressants (i.e., tacrolimus, cyclosporine, and sirolimus), and its serum through concentration is monitored 5–7 days into the therapy.
Among triazoles, isavuconazole is a broad-spectrum antifungal agent for the treatment of invasive aspergillosis (200 mg q 8 h for 6 doses, then 200 mg daily).144–146 Even though isavuconazole and posaconazole show fewer DDIs than voricanozole, posaconazole retain DDI with tacromilus and serum concentrations of both drugs should be carefully monitored.144,145
CandidaCandida infection usually manifests as candidemia and invasive candidiasis following LT. Based on the guidelines published by the American Society of Transplantation Infectious Diseases Community of Practice, the treatment in LTRs is similar to the one prescribed to other patient populations.153
An echinocandin is initially recommended (caspofungin, loading dose 70 mg, then 50 mg IV daily; micafungin, 100 mg IV daily; anidulafungin, 200 mg loading dose, then 100 mg IV daily). When patients are stable and the organism is susceptible, early transition to oral therapy with triazole (e.g. fluconazole) is recommended (Table 1).
CryptococcusCryptococcosis is the third most common invasive fungal infection in SOT recipients. No randomized controlled trials of antifungal therapy for cryptococcosis in SOT recipients have been performed, and treatment recommendations result from the cohorts of HIV-infected patients. According to the Infectious Diseases Society of America, the drug of choice for nonmeningeal cryptococcosis is fluconazole 400 mg per day for 6–12 months (Table 1).154
Treatment recommendations for cryptococcal meningoencephalitis in LTR consist of: i) 2 weeks of induction therapy, liposomal AmB (3–4 mg/kg per day) or amphotericin B lipid complex (ABLC) (5 mg/kg per day) plus flucytosine (100 mg/kg per day); 8 weeks of consolidation therapy, fluconazole (400–800 mg per day); 6 months to 1-year of maintenance therapy, fluconazole (200–400 mg per day).
Pneumocystis jirovecii pneumoniaTrimethoprim-sulfamethoxazole (TMP-SMX) represents the first-line agent and the drug of choice for the treatment of PJP in LTRs (adults: 15–20 mg/kg/day of the TMP component, IV q 6–8 h; children >2 months: 3.75−5 mg/kg/dose of the TMP component and 19–25 mg/kg/dose of the SMX component given IV q 6 h).149 The length of treatment is at least 14 days, and corticosteroids should be associated when pAO2 < 70 mm Hg (adults: 40–60 mg of prednisone equivalent po/iv bid‐tid with tapering after one week over a period of 7–14 days; children: 1 mg/kg po bid for 5 days, then 0.5 mg/kg po bid for 5 days, then 0.5 mg/kg po qd for 10 days).149 Second-choice drugs include alternative agents that are less effective than TMP-SMX and include iv pentamidine, atovaquone, primaquine and clindamycin (Table 1).149
Limitation of the study and conclusionThe major limitation of the present article lies in the narraitve nature of the review compared to those performed systematically. However, the aim of the present study was to provide a concise summary on infectious diseases in lung transplanted patients to guide clinicians dealing with daily issues related to infectious diseases in the immunecompromised hosts. Furthermore, it would be ideal to produce a living document, which is possible for some international guidelines, to update the scientific evidence on the basis of new valuable studies. We could not grade the quality of the evidence and, then, the recommendations, which is usually performed when systematic reviews are carried out. However, the systematic reviews do address specific aims and do not cover a broad range of topics as we did.
In fact, prompt and comprehensive assessment of pre-transplantation infectious comorbidities, both in LT recipient and living donors, from rectal swabs for MDR-colonization to viral serology, and potentially parasitic infections, is needed to decrease the risk of infections and increase the likelihood of patients’ survival. Furthermore, careful assessment of anti-viral and anti-fungal prophylaxis, as well as reasoned empiric antimicrobial treatment,should be tailored on patient's risk factors, microbial colonization, and type of post-transplant immune-soppression.71,116,138
FundingNone. The study is part of the IFALT Working group Activities.
We acknowledge the scientific contribution and support of the IFALT working group. Internal Medicine Department, Division of Infectious Diseases, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico Milano (Milano, Italy): Laura Alagna, Marina Allegrini, Alessandra Bandera, Matteo Bolis, Manuela Carugati, Valentina Ferroni, Andrea Gori, Teresa Itri, Davide Mangioni, Debora Mondatore, Valeria Pastore, and Federica Portunato. Internal Medicine Department, Respiratory Unit and Adult Cystic Fibrosis Center, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico Milano (Milano, Italy): Stefano Aliberti, Francesco Blasi, Letizia Corinna Morlacchi, Martina Oriano, Valeria Rossetti, Paolo Tarsia, and Leonardo Terranova. Thoracic Surgery and Lung Transplant Unit, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico Milano (Milano, Italy): Rosaria Carrinola, Francesco Damarco, Paolo Mendogni, Mario Nosotti. Alessandro Palleschi, Ilaria Righi, Lorenzo Rosso, and Davide Tosi. Pathology Department, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico Milano (Milano, Italy): Stefano Bogetto Ferrero. Department of Biomedical and Clinical Sciences, Università degli Studi di Milano (Milano, Italy): Mario Clerici, Claudio Fenizia, and Daria Trabattoni. Department of Oncology and Hemato-Oncology, Università degli Studi di Milano (Milano, Italy): Claudia Alteri and Carlo Federico Perno. Genprobio srl: Marco Ventura and Claudio Pessina. Prossima Isola srl: Daniele Idini.
Tha authors would like to thank Emma Dempsey for the English language revision.