Obstructive sleep apnea syndrome (OSAS) and insomnia often coexist, and it is estimated that nearly half of those who suffer from the former report symptoms of the latter. The fact that these patients have no other causes of insomnia indicates that it is a sign of OSAS.
ObjectiveThe aim of the study is to evaluate the effectiveness of nocturnal ventilatory support (NVS) in the treatment of insomnia secondary to OSAS.
Materials and methodsIn order to conduct the retrospective study, the authors reviewed the medical records of patients with insomnia and OSAS that had received NVS. Patients with psychiatric disorders, sleep movement disorders, psycho-physiological insomnia, circadian rhythm sleep disorders, inadequate sleep hygiene, use and abuse of hypnotic agents, stimulants, antidepressants, anxiolytics and alcohol, were excluded. For the selected patients, the effects of NVS in terms of clinical signs and symptoms of insomnia, apnea–hypopnea index (AHI), Epworth Sleepiness Scale (ESS) score, and number of sleep hours were analyzed, before and after treatment with NVS.
ResultsAfter reviewing 1241 medical records, 56 patients were selected, with a mean age of 60.9±10.0 years. Twenty-two (39.3%) suffered from intermediate insomnia, 19 (33.9%) had initial insomnia, eight (14.3%) had the mixed type, and seven patients (12.5%) had terminal insomnia. The majority of patients (n=48; 85.7%) were treated with auto-titrating continuous positive airway pressure (APAP). Forty-four patients (78.6%) overcame insomnia; insomnia symptoms persisted in nine (16.1%), and three (5.4%) patients abandoned during the medical follow-up. There was an association between the type of insomnia and its resolution and, in percentage terms patients with the mixed type did not manage to overcome insomnia symptoms (75%).
There was a statistically significant difference between patients that overcame insomnia and those who did not in terms of the average time which elapsed between the initiation of treatment with NVS and compliance with the adherence criteria: 161±61 days for the former, and 225±141 days for the latter (p=0.003). Before and after the NVS treatment, patients slept an average of 5.29±1.37 and 6.37±1.55h per night, respectively (p<0.001). Among the patients who overcame insomnia, six did not meet the treatment adherence criteria: five adhered more than 4h/night in less than 70% of all nights (60.6±3.2%), and one patient adhered less than 4h in all nights (3.5h/night).
ConclusionNVS has proved effective in treating insomnia secondary to OSAS, and favorable results could be observed even in patients that did not meet the criteria of NVS adherence. Results suggest that all insomnia subtypes, except the mixed subtype, may derive from OSAS.
Insomnia is the most common sleep disorder, and is estimated to affect 33–50% of the adult population.1 It can manifest itself either as a primary entity or as secondary to various causes. Obstructive sleep apnea syndrome (OSAS) is the second most common sleep disorder, and can be found in 4% men and 2% women.2 Although they can be perceived as opposite conditions (insomnia characterized by excessive alertness and OSAS by hypersomnolence), it has been observed that they often coexist. Previous studies have shown that 40–50% patients diagnosed with OSAS mentioned significant symptoms of insomnia.3–5 Other studies assessed the presence of OSAS in individuals with insomnia, observing a OSAS prevalence of 29%, 40% and 43%, to cut-off values of the apnea–hypopnea index (AHI) of 15, 10 and 5events/h, respectively.6,7
Published articles about coexisting OSAS and insomnia have approached the issue as the resulting product of two etiologically diverse entities that influence one another. Chung,8 on the contrary, has suggested that in some cases insomnia is a potential symptom of OSAS, manifesting itself secondarily to micro-awakenings. Based on these premises, the authors intend to assess the efficiency of nocturnal ventilatory support (NVS) in treating insomnia which is secondary to OSAS.
MethodsA review was conducted on the clinical files of patients diagnosed with OSAS and insomnia, who were then treated with NVS. Signs indicating insomnia were: sleep latency of 30min or more; waking up after falling asleep and difficulty in falling asleep again for 30min or more, or premature morning awakening for more than 30min, more than three times a week. The use of the Sleep Disorders Questionnaire (SDQ) in the first consultation complemented the clinical interview on identifying patients with insomnia, and also provided information about sleep time before NVS. Epworth sleepiness scale (ESS) was evaluated as well. The OSAS diagnosis was obtained by performing a cardiorespiratory sleep study and/or a polysomnography, which allowed us to classify OSAS severity as mild (AHI: 5–15/h), moderate (AHI: 16–30/h) and severe (AHI>30/h).9 Patients presenting potentially insomnia inducing disorders (anxiety, depression, psychophysiological insomnia, restless legs syndrome, periodic limb movement disorder, altered circadian rhythm, substance abuse, stimulants, antidepressants, anxiolytics, sedatives and hypnotic drugs, inadequate sleep hygiene, medical condition and/or environmental factors that disturb sleep) were excluded.
All patients were treated with NVS. Subsequent consultations were held every 3–4 months, until any problems were resolved. The symptoms of insomnia were reassessed through clinical questionnaires, as well as the ESS. The ventilator records were also taken into account. Sleep time after NVS was inferred from the ventilator adherence records. Patients were considered adherent to NVS treatment if complying with more than 4h a night in, at least, 70% of nights.10
The statistical analysis was performed with SPSS statistical software, version 17 (Chicago, IL). The statistical descriptions of the variables included mean and standard deviation for continuous variables, and absolute and percentage frequency for each group of a categorical variable. Comparisons between two groups were performed with the t-Student's test for independent samples, and with variance analysis (ANOVA) for more than two groups. Values p<0.05 were considered statistically significant.
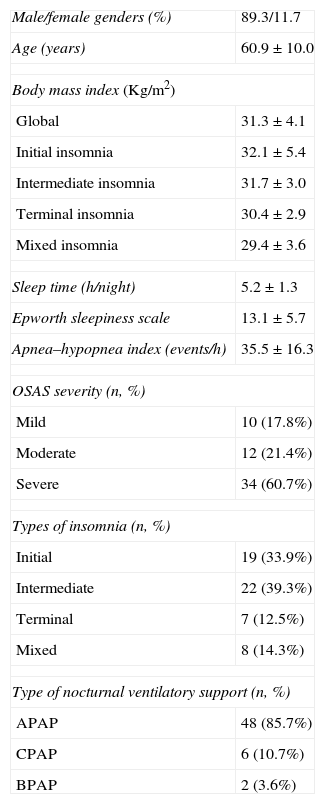
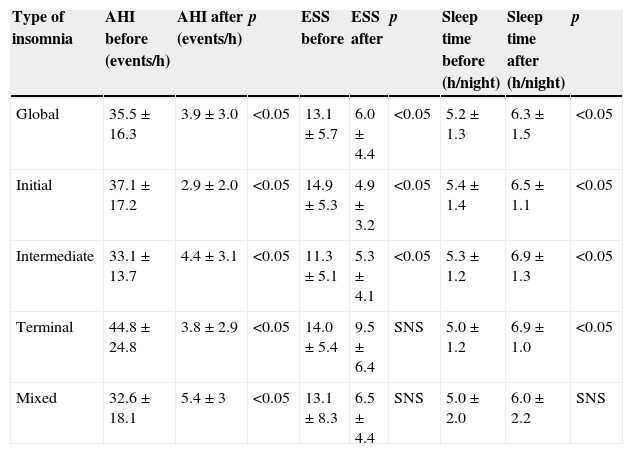
ResultsAfter reviewing 1241 clinical files, 56 patients were selected (4.5%); their characteristics are detailed in Table 1. The four insomnia subtypes did not present statistically significant differences in terms of body mass index (BMI) (Table 1) and initial AHI (AHI before treatment, Table 2). In relation to ESS (Table 2), it was verified that patients with initial insomnia had a pre-treatment ESS which was significantly higher than that of patients with intermediate insomnia (14.9±5.3 vs. 11.3±5.1; p=0.028).
Characteristics of the studied population (n=56).
| Male/female genders (%) | 89.3/11.7 |
| Age (years) | 60.9±10.0 |
| Body mass index (Kg/m2) | |
| Global | 31.3±4.1 |
| Initial insomnia | 32.1±5.4 |
| Intermediate insomnia | 31.7±3.0 |
| Terminal insomnia | 30.4±2.9 |
| Mixed insomnia | 29.4±3.6 |
| Sleep time (h/night) | 5.2±1.3 |
| Epworth sleepiness scale | 13.1±5.7 |
| Apnea–hypopnea index (events/h) | 35.5±16.3 |
| OSAS severity (n, %) | |
| Mild | 10 (17.8%) |
| Moderate | 12 (21.4%) |
| Severe | 34 (60.7%) |
| Types of insomnia (n, %) | |
| Initial | 19 (33.9%) |
| Intermediate | 22 (39.3%) |
| Terminal | 7 (12.5%) |
| Mixed | 8 (14.3%) |
| Type of nocturnal ventilatory support (n, %) | |
| APAP | 48 (85.7%) |
| CPAP | 6 (10.7%) |
| BPAP | 2 (3.6%) |
APAP: autotitrating continuous positive airway pressure; BPAP: bi-level positive airway pressure; CPAP: single continuous positive airway pressure; OSAS: obstructive sleep apnea syndrome.
Evolution of AHI and ESS parameters and sleep time after NVS adaptation, globally and by insomnia subtype.
| Type of insomnia | AHI before (events/h) | AHI after (events/h) | p | ESS before | ESS after | p | Sleep time before (h/night) | Sleep time after (h/night) | p |
|---|---|---|---|---|---|---|---|---|---|
| Global | 35.5±16.3 | 3.9±3.0 | <0.05 | 13.1±5.7 | 6.0±4.4 | <0.05 | 5.2±1.3 | 6.3±1.5 | <0.05 |
| Initial | 37.1±17.2 | 2.9±2.0 | <0.05 | 14.9±5.3 | 4.9±3.2 | <0.05 | 5.4±1.4 | 6.5±1.1 | <0.05 |
| Intermediate | 33.1±13.7 | 4.4±3.1 | <0.05 | 11.3±5.1 | 5.3±4.1 | <0.05 | 5.3±1.2 | 6.9±1.3 | <0.05 |
| Terminal | 44.8±24.8 | 3.8±2.9 | <0.05 | 14.0±5.4 | 9.5±6.4 | SNS | 5.0±1.2 | 6.9±1.0 | <0.05 |
| Mixed | 32.6±18.1 | 5.4±3 | <0.05 | 13.1±8.3 | 6.5±4.4 | SNS | 5.0±2.0 | 6.0±2.2 | SNS |
ESS: Epworth sleepiness scale; AHI: apnea/hypopnea index; SNS: statistically non-significant; p: p-value (values less than 0.05 were considered statistically significant).
Complaints of insomnia were resolved in 44 patients (78.6%), and persisted in 9 (16.1%); 3 (5.4%) abandoned the follow-up. Overall, the time required for compliance with NVS treatment was of 171±84 days. Among patients who overcame insomnia, 41 (93.2%) proved to be compliant with NVS and reported resolution of insomnia complaints simultaneously in the same subsequent appointment (after 150±63 days of treatment). In three cases, there was a discrepancy of 120±51 days between the appointment in which the compliance with treatment was targeted (after 165±54 days of treatment), and the appointment in which the insomnia complaints were reported as solved (after 285±65 days of treatment).
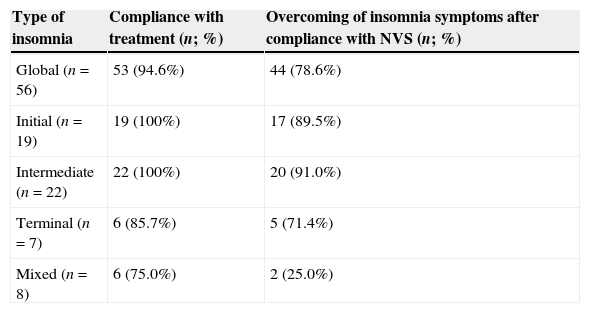
Compliance with NVS by subtype of insomnia and effects on insomnia symptoms are presented in Table 3. The majority of patients presenting with the mixed type did not overcome insomnia (75%). On the other hand, the majority of patients presenting with initial, intermediate and terminal insomnia were successful in overcoming their complaints (89.5%, 91% and 71.4%, respectively; =0.001) (Table 3). Seven patients did not totally fulfill adherence criteria: six adhered more than 4h/night in less than 70% of all nights (61.0±3.0%), and one patient adhered less than 4h in all nights (3.5h/night). However, only one of these did not solve insomnia symptoms.
Compliance with NVS and effects on insomnia symptoms.
| Type of insomnia | Compliance with treatment (n; %) | Overcoming of insomnia symptoms after compliance with NVS (n; %) |
|---|---|---|
| Global (n=56) | 53 (94.6%) | 44 (78.6%) |
| Initial (n=19) | 19 (100%) | 17 (89.5%) |
| Intermediate (n=22) | 22 (100%) | 20 (91.0%) |
| Terminal (n=7) | 6 (85.7%) | 5 (71.4%) |
| Mixed (n=8) | 6 (75.0%) | 2 (25.0%) |
NVS: nocturnal ventilatory support.
In relation to the performance of the various NVS modes out of all the patients undergoing APAP 77.1% have solved insomnia, 16.6% have not, and 6.25% quit; every patient undergoing CPAP has solved insomnia; and of the two patients undergoing BPAP, one has solved insomnia and the other has not. The mean values of pressure on 90% nighttime (P90) and fixed pressure were 9.66±2.84cm H2O in patients who overcame insomnia, and 10.08±3.90cm H2O in patients who did not (p=0.763).
There was an overall decrease in AHI (35.5±16.3–3.9±3.0events/h; p<0.001) and ESS (13.1±5.7–6.0±4.4; p<0.001) under NVS. Sleep time increased significantly after introducing NVS (from 5.2±1.3 to 6.3±1.5h/night; p<0.001). The effects of NVS globally and per insomnia subtypes, are displayed in Table 2.
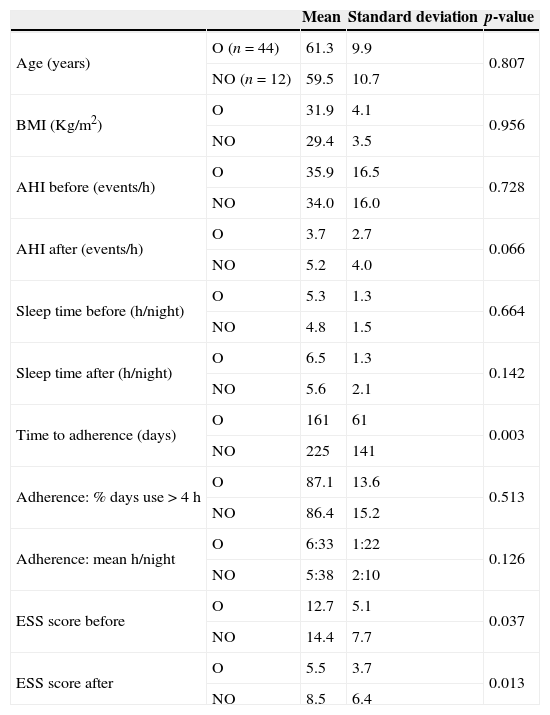
A statistically significant difference was found between the group of patients that overcame insomnia and the group of those who did not, regarding the average time required for compliance with the adherence criteria (161±61 vs. 225±141 days; p=0.003) (Table 4). Groups also presented statistically significant differences in relation to pre- and post-treatment ESS, with the group of patients that did not solve insomnia complaints showing higher scores (Table 4).
Initial parameters: comparison between the group of patients that overcame insomnia (O) and the group of patients that did not (NO).
| Mean | Standard deviation | p-value | ||
|---|---|---|---|---|
| Age (years) | O (n=44) | 61.3 | 9.9 | 0.807 |
| NO (n=12) | 59.5 | 10.7 | ||
| BMI (Kg/m2) | O | 31.9 | 4.1 | 0.956 |
| NO | 29.4 | 3.5 | ||
| AHI before (events/h) | O | 35.9 | 16.5 | 0.728 |
| NO | 34.0 | 16.0 | ||
| AHI after (events/h) | O | 3.7 | 2.7 | 0.066 |
| NO | 5.2 | 4.0 | ||
| Sleep time before (h/night) | O | 5.3 | 1.3 | 0.664 |
| NO | 4.8 | 1.5 | ||
| Sleep time after (h/night) | O | 6.5 | 1.3 | 0.142 |
| NO | 5.6 | 2.1 | ||
| Time to adherence (days) | O | 161 | 61 | 0.003 |
| NO | 225 | 141 | ||
| Adherence: % days use>4h | O | 87.1 | 13.6 | 0.513 |
| NO | 86.4 | 15.2 | ||
| Adherence: mean h/night | O | 6:33 | 1:22 | 0.126 |
| NO | 5:38 | 2:10 | ||
| ESS score before | O | 12.7 | 5.1 | 0.037 |
| NO | 14.4 | 7.7 | ||
| ESS score after | O | 5.5 | 3.7 | 0.013 |
| NO | 8.5 | 6.4 |
AHI: apnea/hypopnea index; BMI: body mass index; ESS: Epworth sleepiness scale; OSAS: obstructive sleep apnea syndrome.
Among patients who continued to have insomnia symptoms, none worsened complaints; instead: (a) three changed the default; (b) two reported subjective improvement in symptoms, but kept insomnia criteria; (c) four still had complaints, without improvement or worsening. One patient was referred to a psychologist, after an anxiety profile was identified. No cause for insomnia was identified regarding the remaining patients.
DiscussionIn this work, we have observed favorable results regarding symptoms of insomnia in patients with OSAS that were treated with NVS. AHI and ESS have also evolved favorably.
Previous studies have proved that patients with OSAS and insomnia showed improvement in terms of insomnia complaints and daytime sleepiness as a response to OSAS related therapeutic approaches, such as the use of CPAP,11 surgical treatment of OSAS,12 or the applying of nasal dilator strips.13
One of the differences between our work and others who have evaluated the relationship between OSAS and insomnia subtypes is the criteria used to choose patients. We have excluded patients with other identifiable causes for insomnia, in an attempt to select patients with symptoms secondary to OSAS; the other studies evaluated both OSAS and comorbid insomnia whatever the cause of insomnia.
In spite of using different criteria for selection, in a similar way to previous studies,8,14 the most frequent insomnia subtype in our study was the intermediate (39.3%).
Chung and co.8 have proposed a causal relationship between intermediate insomnia and OSAS, with the multiple microawakenings secondary to repeated apneas resulting in sleep fragmentation and, thus, symptoms of intermediate insomnia. The same authors have also verified that patients with OSAS and intermediate insomnia were more obese, had higher AHI and higher ESS score than patients with OSAS and initial insomnia.8,14 On the other hand, patients with OSAS and initial insomnia were not sleepier than patients with OSAS and no insomnia, which is consistent with the hyperarousal state in patients with insomnia. Bjornsdottir and co. (2013) have not only verified a higher adherence to PAP by patients with intermediate insomnia, but also a significant improvement in complaints under treatment, once more favoring a causal relationship between OSAS and intermediate insomnia. On the contrary, it can be observed in the same study that there is not only a negative impact of initial insomnia regarding adherence to PAP, but also a lack of a significant improvement in complaints under PAP.15
Contrary to the above mentioned studies, in our work insomnia subgroups did not present significant differences regarding both BMI and basal AHI. The ESS score was higher in patients with initial insomnia than in those with intermediate insomnia. We also verified a significant adherence to treatment not only by patients with intermediate insomnia, but also by patients with initial and terminal insomnia, with adherence rates higher than 80%. Only mixed insomnia patients had a lower adherence rate. The treatment's success rates regarding the resolution of insomnia complaints were equally important for all insomnia subtypes, except for the mixed subtype. The fact that we have made an attempt to select only cases secondary to OSAS may explain that not only intermediate insomnia, but also the initial and terminal subtypes presented high BMI, AHI and ESS values, typical of OSAS, as well as good adherence to NVS and good results. In this perspective, and considering that insomnia is characteristically connected with a state of hyperarousal, every patient with insomnia and excessive daytime sleepiness should arouse suspicion of associated OSAS, namely as a cause for insomnia itself.
An analysis by insomnia subtype has shown that the majority of patients with mixed insomnia did not overcome the symptoms of insomnia, despite an improvement in AHI, ESS and sleep time. These results suggest that all subtypes were related to OSAS, except mixed insomnia.
Why did some patients maintain insomnia complaints, despite the efforts to eliminate other possible causes for insomnia? An explanation to consider would be the existence of a true insomnia secondary to OSAS in these patients, solved after treating obstructive events with NVS; originating, however, insomnia secondary to the use of NVS itself, as reported in the study by Mota et al. (2011).16 In this study, most patients with insomnia de novo were being treated with APAP. Previous studies have suggested that pressure variation during the night could be the reason for sleep fragmentation, and, as a consequence, insomnia complaints in patients undergoing APAP.17,18 During our study, all patients that kept insomnia after beginning NVS were under APAP, except one patient with BPAP. Another possibility would be the presence of exclusion diagnostics, harder to identify, such as idiopathic insomnia, paradoxical insomnia, or even changes in the circadian rhythm. There is also the fact that patients were not evaluated by mental health specialists, or through specific questionnaires for depression and anxiety evaluation. These issues may have allowed for subtle psychological factors to remain unidentified. Still, even admitting that some patients may have escaped the attempted exclusion of cases of insomnia not secondary to OSAS, the obtained results really do seem to support our initial hypothesis.
A comparison between the group of patients that solved insomnia and the group of patients that did not has shown that the adaptation of the latter was significantly slower, and required higher airway pressures. Moreover, patients that did not solve insomnia presented higher residual AHI than patients that solved insomnia. This finding may justify the need for higher airway pressures, greater discomfort and, as a consequence, more time to adapt. These patients also presented a higher pre- and post-treatment ESS when compared to patients that solved insomnia. The higher post-treatment ESS could be justified by the fact that the respiratory events were not completely solved (residual AHI: 5.2±4.0/h), although there still seems to be a great discrepancy between residual AHI and daytime sleepiness complaints. Could a higher pre- and post-treatment ESS be connected with a different insomnia etiology in patients that failed to solve it? But, if it is indeed insomnia not associated with OSAS, since it did not improve with NVS, would it not be expected that the ESS be lower than that of patients that have solved insomnia, thus reflecting a hyperarousal state? The follow-up of patients did not allow clarification of these doubts, and more research is needed in this particular subject area.
It has also been observed that even though they did not meet the criteria of NVS adherence (4h on 70% of nights), six patients overcame insomnia. These data suggest that a reduced use of NVS may yield therapeutic benefits as well.
ConclusionNVS has proved effective in treating insomnia secondary to OSAS, and favorable results were observed even in patients that did not meet the criteria of NVS adherence. Results suggest that all insomnia subtypes, except the mixed subtype, may derive from OSAS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.