A 47-year-old male was admitted with subacute onset of dry cough and fever. Chest tomography demonstrated multifocal areas of consolidation and ground glass attenuation. Cytological analysis of bronchoalveolar lavage revealed lymphocytosis and eosinophilia and anatomopathological exam of transbronchial cryobiopsy showed poorly formed non-caseous granulomas associated to interstitial lympho-plasmocitary infiltrate. The diagnosis of idiopathic granulomatous lung disease (GLD) was assumed and the patient started oral prednisolone, presenting clinical, functional and radiological improvement. Two years later, the patient was diagnosed with primary biliary cirrhosis (PBC). At this time, it was possible to associate GLD with the autoimmune hepatobiliary disease.
Clinical, epidemiological and pathological aspects of this uncommon case of interstitial lung disease as first presentation of PBC in a male patient are discussed.
Primary biliary cirrhosis (PBC) is a chronic autoimmune disorder of unknown etiology. It affects predominantly middle-aged women and is characterized by progressive immune-mediated destruction of the small- and medium-sized intrahepatic bile ducts causing cholestasis and cirrhosis.1–3 PBC is often associated to other autoimmune diseases1,4,5 and figures prominently among the primary autoimmune hepatobiliary diseases (AHD) with pulmonary involvement.5 Cases of coexistent PBC and subclinical alveolitis, lymphoid interstitial pneumonia, organizing pneumonia, interstitial fibrosis, nonspecific interstitial pneumonia, granulomatous lung disease (GLD)5,6 and, more recently, acute fibrinous and organizing pneumonia7 have been described. The prevalence of GLD related to PBC1,8,9 is not known, although it seems to be rare. In all reported cases, pulmonary sarcoid reaction was diagnosed during the course of PBC in women. Alternatively, interstitial lung disease (ILD) as the initial manifestation of PBC in males, to the best of the author's knowledge, has not been previously reported, and its clinical features are unknown.
This is a case of a male patient who first developed GLD and then met diagnostic criteria of PBC which later was present and the literature about this topic is discussed.
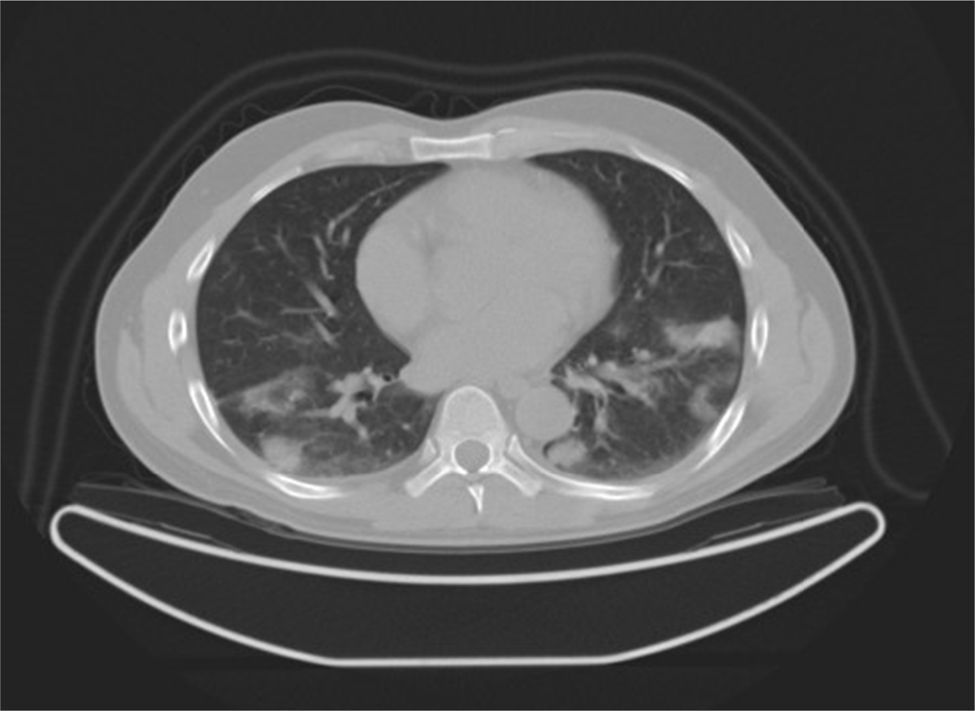
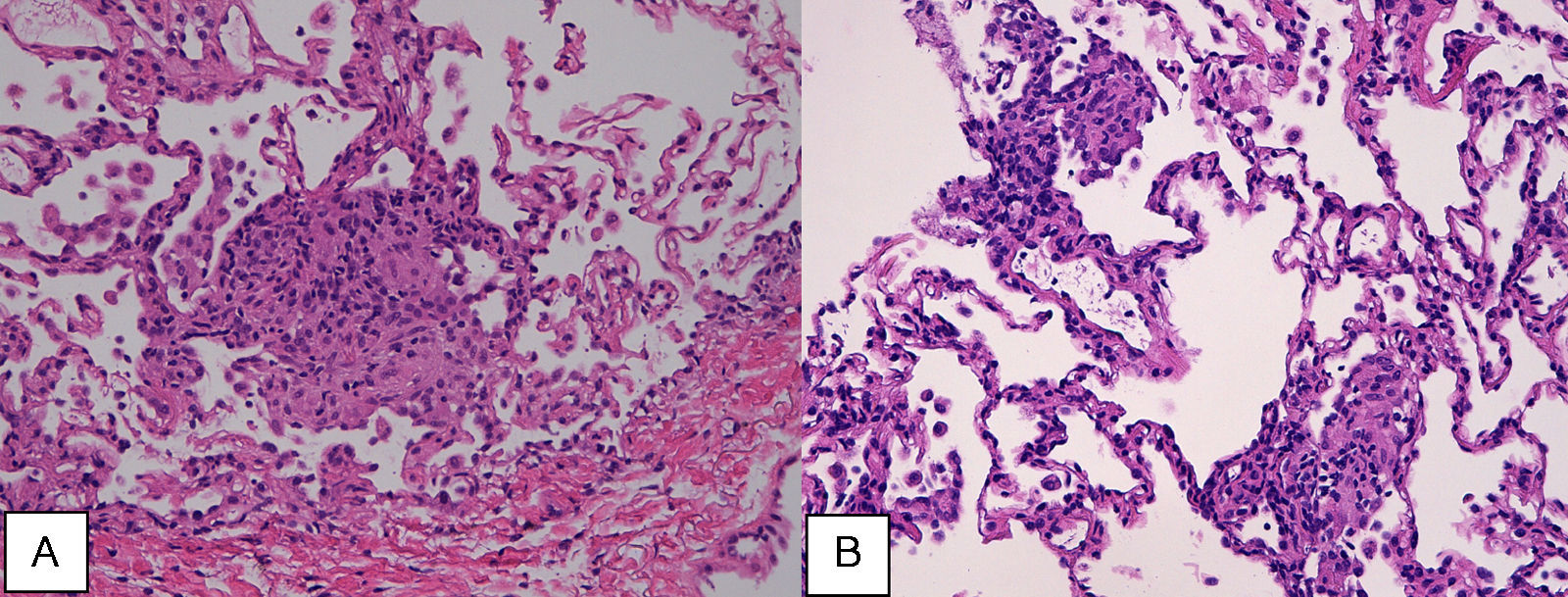
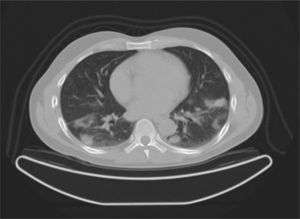
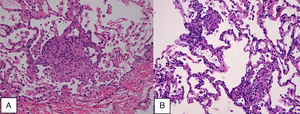
Case reportA 47-year-old male, office worker, smoker (23 pack-years) and without comorbidities, presented with subacute onset of fatigue, nonproductive cough and fever. He had been an emigrant in South America 10 years ago. Pulmonary auscultation revealed bilateral crackles in lower zones, otherwise the physical examination was unremarkable. Laboratory tests showed eosinophilia (850/μL) and mild elevation of C-reactive protein (7.9mg/dL). Bilateral opacities were observed in chest radiography and chest computed tomography, subsequently performed, showed multifocal bilateral and peripheral areas of consolidation and ground glass with perilobular pattern and halo sign (Fig. 1). Arterial blood gases were within normal range. Signs or symptoms of connective tissue disease or drug toxicity were absent and exposure history was negative. Immunological study revealed only positive antimitochondrial antibody (AMA). Due to that result and in order to exclude autoimmune liver disease, liver ultrasound was performed revealing no changes. Bronchoscopy also showed no macroscopic changes. Bronchoalveolar lavage (BAL) of right B9 segment was performed and cytological analysis revealed normal total cell count for a smoker (320×106/L), with 35% lymphocytes, 10% neutrophils, 3% eosinophils, 52% macrophages and normal CD4/CD8 ratio of 1.9. Microbiological analysis of BAL fluid was negative. Due to eosinophilia and his previous history of emigration in South America, the hypothesis of chronic pulmonary paragonimiasis was considered but research of Paragonimus westermani on BAL was negative. Transbronchial cryobiopsy of right B9 was carried out and histological examination showed poorly formed non caseous granulomas occupying terminal bronchioles and alveolar space, associated to interstitial lympho-plasmocitary infiltrate (Fig. 2). Values of pulmonary function testing were within the normal range, except DLCO (66%) that was slightly decreased. The diagnostic hypothesis of idiopathic GLD was assumed and the patient started oral prednisolone (40mg/day) therapy, presenting clinical and functional (DLCO 79%) improvement and resolution of pulmonary consolidations.
Two years after ILD diagnosis, and still under oral prednisolone (10mg/week), the patient presented fatigue, pruritus and biochemical evidence of cholestasis. Ultrasonography of liver showed signs of cirrhosis. The diagnosis of PBC was made considering positive AMA and the cholestatic pattern of liver enzymes, according to the criteria proposed by American Association of Study of Liver Diseases.21 Ursodeoxycholic acid (13mg/kg/day) was started. At this time it was possible to associate ILD with the primary autoimmune hepatobiliary disease (AHD).
DiscussionAHD carries the potential to induce or be associated with ILD and PBC figures prominently among the primary AHD with pulmonary involvement, maybe because it is the most frequent and widely studied.5 In a prospective study, 15.7% of PBC patients had concomitant ILD and 53.6% of them had respiratory symptoms.6 There is increasing evidence of autoimmune pathophysiology occurring within the hepatopulmonary axis that may lead to clinically evident disease.5 Coexistent extrahepatic autoimmune disease arises in 70–85% of PBC patients,5,10 with 41% having more than 1 concurrent autoimmune process.5 Shen et al.6 demonstrated that Raynaud phenomenon and association with other connective tissue diseases were risk factors for PBC patients to develop ILD.
The diagnosis of PCB must fulfill at least 2 out of 3 of the following criteria: (1) chronic cholestasis with elevated alkaline phosphatase and/or gamma-glutamyl transpeptidase for at least 6 months, (2) the presence of serum AMA (titer≥1:40), and (3) a histopathological study of a liver specimen biopsy indicating PCB.3 In this case, although liver biopsies were not performed, the presence of AMA and biochemical evidence of cholestasis established the diagnosis of PBC. Serum AMA are widely accepted as the diagnostic hallmark of PBC, found in 90–95% of patients and less than 1% of normal controls, with a very high specificity,3,11 and may precede disease onset by several years,2 as in the case here described. Metcalf et al.12 showed that before the advent of any clinical or biomedical criteria, individuals positive for AMA do have PBC. In this setting, it seems right that patients with interstitial lung disease, positive AMA and absence of liver dysfunction, should have their liver function tests monitored, given the high probability of developing PBC.
PBC overwhelmingly affects females, with a female to male ratio estimated as 10 to 1.2 Epidemiological studies estimate that approximately 7–11% of PBC patients are male.13 The reasons underlying the low incidence of PBC in males are largely unknown. A role of fetal microchimerism, sexual hormones or X chromosome linked defects has been proposed.2 There does not appear to be any histological, serological, or biochemical differences between male and female PBC, although the symptoms may differ, with males being at higher risk of life-threatening complications such as gastrointestinal bleeding and hepatocellular carcinoma and autoimmune associated conditions being more common in female.13,14
The major noninfectious causes of GLD are sarcoidosis, hypersensitivity pneumonitis, granulomatosis with polyangiitis, aspiration pneumonia, common variable immunodeficiency, berylliosis, drug-induced lung diseases and talc granulomatosis.15–18 GLD is rarely found in association with PBC and to date, all the published clinical reports have described cases of female gender.1,8,9 It is not uncommon to observe hepatic noncaseating epithelioid granulomas in PBC, particularly in early-stage disease,19 suggesting a similar pathway on the hepatic inflammatory activity of PBC and pulmonary granulomatous damage. Wallaert et al.20 demonstrated that subclinical alveolar inflammation comprising T-lymphocytes and activated alveolar macrophages mimicking sarcoid alveolitis is present in a high proportion of PBC patients. Also Spiteri et al.21 suggested that the immune mechanisms involved in the formation of granulomas are similar to that of sarcoidosis. In this case, despite lymphocytic alveolitis, radiologic findings were not suggestive of sarcoidosis (no mediastinal adenopathies or small nodules were present), granulomas were poorly differentiated (unlike the well-formed granulomas of sarcoidosis) and CD4/CD8 ratio was normal, making the diagnosis of pulmonary sarcoidosis less probable.
Some authors described cases of ILD preceding connective tissue disease as rheumatoid arthritis and Sjögren's syndrome.22–24 The time interval from prodromal respiratory symptoms to connective tissue disease ranged between 0.5 and 24 months in the cases of rheumatoid arthritis and 36 months in the patient with Sjögren's syndrome. In our patient, GLD preceded PBC diagnosis by around 24 months. It is not known whether treatment with corticosteroids masked or delayed the onset of liver disease.
The data on the treatment of the lung disease in the course of PBC are limited.1 GLD seems to be a steroid responsive condition. In all reported cases of GLD associated to PBC, and also in this case, clinical and radiological improvements were obtained with corticosteroid therapy. Apart from corticosteroids, other immunosuppressive medications, i.e. azathioprine, cyclophosphamide, methotrexate and cyclosporine, have also been used.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors declare that there are no conflicts of interest.