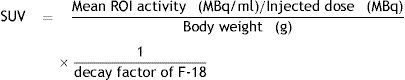Although a number of studies in patients with a variety of malignant tumors have shown that metabolic activity on fluorine-18 deoxyglucose positron emission tomography computed tomography (18F-FDG-PET/CT) is correlated with survival, there are few studies about the impact of 18F-FDG-PET/CT for survival in small cell lung cancer (SCLC) patients. There is still some ambiguity as to whether FDG PET in patients with SCLC will ensure prognostic knowledge for survival. We performed a retrospective analysis of prognostic implication of 18F-FDG-PET/CT in patients with SCLC.
MethodsWe retrospectively reviewed 54 patients with histologically or cytologically proven SCLC who had undergone pre-treatment 18F-FDG-PET/CT scanning between September 2007 and November 2011 in the Dicle University, School of Medicine, Department of Medical Oncology. SUVmax and other potential prognostic variables were chosen for analysis in this study. Univariate and multivariate analyses were conducted to identify prognostic factors associated with survival.
ResultAmong the eleven variables of univariate analysis, three variables were identified as having prognostic significance: Performance status (p<0.001), stage (p=0.02) and diabetes mellitus (p=0.05).
Multivariate analysis showed that performance status and stage were considered independent prognostic factors for survival (p<0.001 and p=0.002 respectively).
ConclusionIn conclusion, performance status and stage were identified as important prognostic factors, while 18F-FDG-PET/CT uptake of the primary lesions was not associated with prognostic importance for survival in patients with SCLC.
Embora uma série de estudos em pacientes com uma diversidade de tumores malignos tenham demonstrado que a atividade metabólica na tomografia computorizada por emissão de positrões de deoxiglucose marcada com flúor-18 (18F-FDG-PET/CT) está correlacionada com a sobrevivência, existem poucos estudos sobre o impacto do 18F-FDG-PET/CT para a sobrevivência em pacientes com cancro pulmonar de células pequenas (SCLC). Ainda existe alguma ambiguidade de que, em pacientes com SCLC, o FDG PET forneça informações importantes relativamente de prognóstico para a sobrevivência. Realizámos uma análise retrospetiva da implicação no prognóstico de 18F-FDG-PET/CT em pacientes com SCLC.
MétodosAnalisámos retrospetivamente 54 pacientes com SCLC comprovado histologicamente ou citologicamente, que tinham realizado 18F-FDG-PET/CT entre setembro de 2007 e novembro de 2011, na Universidade de Dicle, Faculdade de Medicina, Departamento de Oncologia Médica. Foram escolhidas a SUVmax e outras potenciais variáveis de prognóstico para a análise neste estudo. Foram realizadas análises univariadas e multivariadas para identificar os fatores de prognóstico associados à sobrevivência.
ResultadoEntre as 11 variáveis da análise univariada, 3 variáveis foram identificadas como tendo significância para o prognóstico. Estado Geral (p<0,001), estádio (p=0,02) e diabetes mellitus (p=0,05).
A análise multivariada mostrou que o Estado Geral e o estádio foram considerados fatores de prognóstico independentes para a sobrevivência (p<0,001, p=0,002 respetivamente).
ConclusãoEm conclusão, o Estado Geral e o estádio foram identificados como importantes fatores de prognóstico, enquanto a absorção de 18F-FDG-PET/CT das lesões primárias não se associou ao prognóstico para a sobrevivência em pacientes com SCLC.
Lung cancer is the most common among cancer-related deaths in both men and women in worldwide. Small cell lung cancer (SCLC) represents approximately 15% of all diagnosed lung cancers cases.1,2 SCLC is associated with a more rapid tumor doubling time, a high growth fraction and early widespread dissemination. As a result of this, overall survival (OS) rates for these patients are disappointingly low.
The Veterans Administration Lung Study Group two-tiered staging system was used to classify SCLC as either limited disease (LD) or extended disease (ED) which was primarily based on compatibility for treatment options.3 Despite its practical usefulness and prognostic advantage, this staging system is not accurate enough to reflect tumor burden, and it is insufficient to predict survival in some patients.
Very different prognostic factors in several trials have been identified for survival in patients with SCLC4–7; however, none of these prognostic factors are sufficiently reliable to base treatment decision on. Even though fluorine-18 deoxyglucose positron emission tomography computed tomography (18F-FDG-PET/CT) scan is widely utilized in staging SCLC, it is not standard work-up for SCLC with respect to international guidelines. Owing to the fact that a number of studies in patients with a variety of malignant tumours8–13 have shown that metabolic activity on 18F-FDG-PET/CT is correlated with survival, there are few studies about the impact of 18F-FDG-PET/CT for survival in SCLC patients.14–17 There remains an ambiguity as to whether 18F-FDG-PET/CT in patients receiving first-line etoposide plus cisplatin (EP) chemotherapy will provide reliable prognostic knowledge about survival.
We performed a retrospective analysis of the prognostic implication of 18F-FDG-PET/CT for patients with SCLC. The aim of this study was to investigate the prognostic significance of the characteristics of patients in SCLC. Specifically, we investigated the prognostic implication of 18F-FDG-PET/CT for OS in the patients receiving first-line EP chemotherapy.
MethodsPatient populationWe retrospectively reviewed 54 patients with histologically or cytologically proven SCLC who had undergone pre-treatment 18F-FDG-PET/CT scanning from September 2007 to November 2011 in the Dicle University, School of Medicine, Department of Medical Oncology. They met the following inclusion criteria; (1) 18 or more years old; (2) a histologic or cytologic diagnosis of SCLC; (3) no previous chemotherapy or radiotherapy; (4) there was sufficient clinical data recorded in medical records; (5) they had to have a measurable disease, as defined by Response Evaluation Criteria in Solid Tumours (RECIST).
Patients were identified as having DM on the basis of elevated fasting glucose level (>126mg/dL), and a history of DM or medication use, such as insulin or oral hypoglycemic agents.
Patients with LD underwent concurrent chemoradiotherapy, which consisted of chemotherapy and thoracic radiotherapy. Both LD and ED patients were receiving first-line EP chemotherapy. The EP regimen consisted of 100mg/m2 etoposide on days 1 and 30mg/m2 cisplatin on days 1–3, every 3 weeks.
All had SCLC. Patients who had received prior treatment were excluded.
FDG-PET imagingFDG-PET was carried out in all cases within 6 weeks before SRBT. All patients fasted for at least 4h before the 18F-FDG-PET/CT examination though oral hydration with glucose-free water was given. When peripheral blood glucose level before administration of 18F-FDG was <150mg/dL, patients received an intravenous injection of 3.70–4.44MBq/kg of FDG. Whole-body FDG-PET was scanned using the same scanner, Biograph 6 PET/CT scanner (CTI/Siemens, Knoxville, TN). The axes of both systems were mechanically aligned so that the patient could be moved from the CT scanner to the PET scanner gantry. The resulting of PET and CT scans co-registered on the same hardware. Then, 1 hour after the injection, CT and PET scans were performed. Images from the level of the middle skull to the proximal thigh were obtained. CT scan was implemented with the following settings: 110kV; 80mA; tube rotation time, 0.8s/rotation per pitch, and section thickness, 3.0mm (whole body CT had 307 or 356 slices). The PET and CT scans were obtained during normal tidal breathing. The PET scans were done immediately after the CT scans. The PET/CT scans were obtained in 3D mode at 3min per bed position.
As a semi-quantitative analysis, the maximum standardized uptake value (SUVmax) was achieved by placing region of interest (ROIs) over the lesions that had been determined as suspicious on visual assessment. SUVmax of the pulmonary tumor was calculated in all cases using a 3D acquisition and the following formula:
The maximum standardized uptake value (SUVmax) was represented by the counts per second of the voxel showing the maximum radioactivity in the volume of interest encompassing the tumor divided by the volume of the voxel (mL).
PET data were iteratively reconstructed using an ordered subset expectation maximization algorithm and segmented attenuation correction (2 iterations, 8 subsets) and the CT data. Co-registered scans were displayed using dedicated software (e-soft-PET; Siemens Medical Solutions).
Two experienced nuclear medicine physicians, who were unaware of the clinical results, viewed and quantitatively analyzed the PET images.
Factors analyzedEleven potential prognostic variables were chosen on the basis of previously published clinical trials. The variables were divided into categories: age (<65 or ≥65), gender (male or female), performance status (PS) (0–1, 2–3), stage (LD or ED), weight loss ≥5% with previous 3 months (present or absent), diabetes mellitus (present or absent), smoking history (present or absent), SUVmax values (<13.0 or ≥13.0), laboratory parameters [(albumin, LDH, blood sugar) (
The values of SUVmax were detected between 5 and 20 in prior studies with log-rank probability values to determine a prognostic cutoff point for SUVmax. Because no statistically significant value was found, SUVmax was dichotomized at its median of 12.9 in present study.
Statistical analysisAll of the analyses were performed using the SPSS statistical software program package (SPSS version 11.5 for windows). The differences in the clinical characteristics between the two groups were analyzed by chi-square test and student t test. Overall survival (OS) was calculated from the start of the first cycle of chemotherapy to the date of death from any cause or the date of the last follow-up. Overall survival was estimated using the Kaplan–Meier method. The Cox proportional hazards regression model was used to determine statistical significant variables related to survival. Differences were assumed to be significant when p value was less than 0.05.
ResultsPatient characteristicsBetween September 2007 and November 2011, 54 patients with SCLC were enrolled in this study. The median age of patients was 57 years (range 28–80) with 50 (92.6%) males and 4 (7.4%) females. The number of patients with a PS score 0–1 was 34 (63.0%). Thirty patients (55.6%) were diagnosed as having extended disease and 24 patients (44.4%) had limited disease. The estimated median OS with LD was 17.3 months (95% CI, 8.9–25.7 months). Median OS of the treated ED patients was 8.4 months (95% CI, 7.1–9.8 months). The patients’ baseline characteristics are listed in Table 1.
The general characteristics of the patients.
| Characteristic | No. of patients (%) |
| Sex | |
| Male | 50 (92.6) |
| Female | 4 (7.4) |
| Age, median (range) | 57 (28–80) |
| Age | |
| <65 | 47 (87.0) |
| ≥65 | 7 (13.0) |
| Performance status | |
| 0–1 | 34 (63.0) |
| 2–3 | 18 (33.3) |
| Unknown | 2 (3.7) |
| Smoking history | |
| Current or former | 46 (85.2) |
| Never | 4 (7.4) |
| Unknown | 4 (7.4) |
| Weight loss | |
| Yes | 34 (63.0) |
| No | 11 (20.4) |
| Unknown | 9 (16.6) |
| Diabetes mellitus | |
| Yes | 40 (74.1) |
| No | 4 (7.4) |
| Unknown | 10 (18.5) |
| Stage | |
| LD | 24 (44.4) |
| ED | 30 (55.6) |
| SUVmax, median | 13 (4.2–29.0) |
| Laboratory parameters, median | |
| Albumin, g/dl | 3.4 |
| LDH, U/l | 248 |
| Blood sugar, mg/dl | 103 |
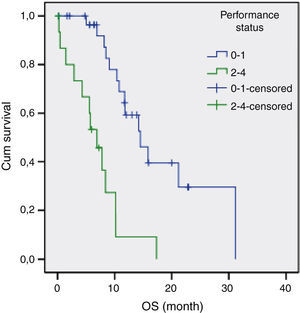
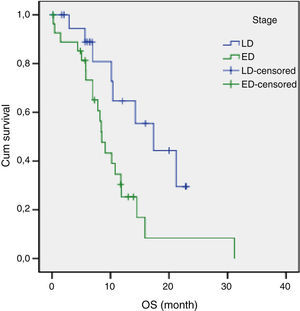
The results of univariate analysis are summarized in Table 2. Among the eleven variables of univariate analysis, three variables were identified as having prognostic significance: Performance status (p<0.001), stage (p=0.02) and diabetes mellitus (p=0.05). Multivariate analysis included the three prognostic significance factors in univariate analysis. The results of multivariate analysis are shown in Table 3. Multivariate analysis by Cox proportional hazard model showed that performance status and stage were considered independent prognostic factors for survival (p<0.001 and p=0.002 respectively) (Figs. 1 and 2).
Univariate analysis of survival time by categorical variable.
| Variable | Log-rank test value | Degrees of freedom | p |
| Sex | 1.16 | 1 | 0.28 |
| Age | 0.29 | 1 | 0.86 |
| Stage | 5.39 | 1 | 0.02 |
| Smoking history | 0.79 | 1 | 0.37 |
| Performance status | 19.2 | 1 | <0.001 |
| Weight loss | 0.1 | 1 | 0.90 |
| Diabetes mellitus | 3.72 | 1 | 0.05 |
| SUVmax, median | 0.18 | 1 | 0.66 |
| Laboratory parameters, median | 1 | ||
| Albumin | 2.45 | 1 | 0.11 |
| LDH | 1.05 | 1 | 0.30 |
| Blood sugar | 1.73 | 1 | 0.18 |
SCLC is very sensitive to radiotherapy and chemotherapy while it is associated with a faster tumor doubling time, a high growth fraction and early widespread dissemination. As a result of this, overall survival rates for these patients are disappointingly low. Patients eligible for chemotherapy should be selected very carefully.
There are a number of studies about this activity as shown on FDG PET in relation to survival in SCLC14–17; the importance of 18F-FDG-PET/CT for survival in patients receiving first-line EP chemotherapy is still subject to controversy. Although Lee et al.15 showed that the degree of SUVmax was strongly associated with an increase in overall survival, Zhu et al.14 and Van der Leest et al.17 on the contrary found no observable prognostic value of SUVmax. In our multivariate analysis, we found that SUVmax value was not a factor associated with survival. The inconsistency of results may be partly explained by blood glucose level of the patient, time to imaging, the biological characteristics of tumor cells and treatment modality.
A poor PS is usually accepted as a negative prognostic factor for all cancer patients.18–20 The importance of PS was also confirmed in advanced SCLC patients.15 Our study showed that poor PS is an associated independent risk factor for survival.
The median survival for limited disease is 14–16 months and only 8–11 months for extensive disease with effective treatment. The overall 5-year survival rate is under 10%.1,21 In previous studies, many authors14,15,17 have shown that the tumor stage at initial presentation was the most important prognostic factor for survival in patients with SCLC. Similarly, stage was found to be an independent prognostic factor of survival in the present study. In our study, the estimated median OS for LD was 17.3 months (95% CI, 8.9–25.7 months) and only 8.4 months (95% CI, 7.1–9.8 months) for ED.
The present study has got some limitations. Firstly, it is based on retrospective studies. Secondly, the number of patients was small. Thirdly, we did not evaluate the type of DM, duration of diabetes and the types of diabetic therapy used. Fourthly, the limit described of 150mg/dl of patient blood glucose level may lower the sensitivity of 18F-FDG-PET/CT. A number of the studies have shown that plasma glucose competes with 18F-FDG uptake of malignant lesions; for this reason it is claimed that hyperglycemia may reduce and impair 18F-FDG uptake of tumors.22–25 However, Mirpour et al.26 and Roy et al.27 showed that the quality of PET/CT images is sufficient to provide a trustworthy clinical opinion, even in those patients with serum glucose level above 180mg/dL. Accordingly, the Society of Nuclear Medicine guidelines for PET/CT advocates that 18F-FDG should not be administered when plasma glucose level is over 150–200mg/dL (8.3–11.1mmol/L).28 The European Association of Nuclear Medicine also recommends that glycemia should ideally not exceed 130mg/dL, and the test should be rescheduled if the serum glucose level is higher than 200mg/dL (7.2mmol/L).29 In fact, there are several points of controversy outstanding. The 18F-FDG tumoural uptake process in hyperglycemia is not yet fully understood.
In conclusion, performance status and stage were identified as important prognostic factors, while FDG uptake of the primary lesions was not associated with the prognostic importance for survival in patients with SCLC. These findings may also facilitate pretreatment prediction of survival and can be used for selecting patients for the correct choice of treatment. Therefore, prospective and larger clinical trials are needed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Inal A, Kucukoner M, Kaplan M, Urakcı Z, Nas N, Guven M, et al. É o 18F -FDG-PET/CT um fator de prognóstico para a sobrevivência em pacientes com cancro pulmonar de pequenas células? Experiência num único centro. Rev Port Pneumol 2013. http://dx.doi.org/.