A seventy-nine-year-old man presented to the emergency department (ED) with dyspnea, cough and bronchial secretions that he was unable to mobilize.
He had a right hemiparesis and dysphagia due to intracerebral haemorrhage, and was a long-term bedridden patient, without cognitive deficits, and with good social support. No previous history of pulmonary diseases.
On admission, he was hemodynamically stable and afebrile, but presented tachypnoea with accessory muscle use. Pulmonary auscultation revealed decreased breath sounds on the left and scattered rhonchi on both sites.
Blood work revealed leucocytosis (24,15 × 10E3/uL leukocytes) and a C Reactive Protein of 3.57 mg/dL. Arterial blood gas showed isolated type 1 respiratory insufficiency (FiO2 35%, pH 7.468, pO2 36.2 mmHg, pCO2 39.9 mmHg, HCO3- 28.7 mmol/L).
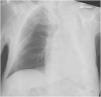
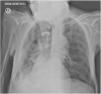
The X ray revealed an opacified white left lung (Fig. 1). Chest tomography scan showed the left airways filled with mucoid material, with atelectasis of the entire left lung and mediastinal shift to the ipsilateral side. Evaluation of the pulmonary parenchyma did not show any suspicious alterations, including consolidations or expansive lesions.
The patient was put on an empiric antibiotic therapy with ceftriaxone 2mg once a day and dual bronchodilation (ipratropium bromide 60 µg every 6 hours, and salbutamol 200 µg every 6 hours).
Since the patient was not deemed eligible for admission to the Intensive care unit (ICU), emergency bronchoscopy was protracted. Instead, and although it wasn't possible to quantify the peak cough flow (PCF) in the ED, the medical team attempted using Mechanical in-exsufflator (MI-E) to clear the tracheobronchial airway.
We used the following parameters for MI-E therapy: oro-nasal interface, automatic mode with cough track, inspiratory pressure of +40 cmH2O for 3 seconds, with low inspiratory flow rate, expiratory pressure of -40cmH2O for 2,5 seconds. We progressively adjusted the pressures with +/- 5cmH2O increments every 2 or 3 cycles, starting with expiration pressure, until +50cmH2O and -60cmH2O, with which we got a MI-E PCF > 280 L/min. The secretions were still hard to expel, so, we added an oscillation on both phases 10Hz, 10cmH2O.
After 8 treatments consisting of 2 series of 5 cycles of MIE, there was a significant increase in vesicular murmur in the left apical region of the thorax and the patient's oxygen saturation increased to 93% with a Ventimask at 31% FiO2. We continued the treatment for an additional two days, six times per day.
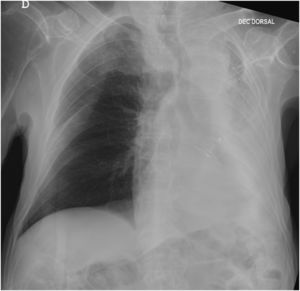
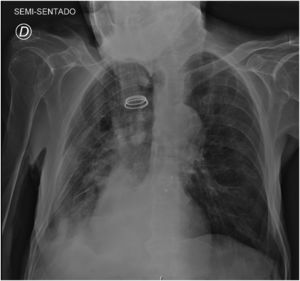
A follow-up Chest X-ray (48h hours later) revealed a significant improvement, with complete resolution of the left lung atelectasis (Fig. 2).
As the patient kept ineffective cough, with PCF less than 60L/min, we instructed the patient's daughter on how to use the MI-E. At discharge, the patient's oxygen saturation was 94% in air.
The elderly and bedridden population is increasing. Neurological diseases and immobility are associated with respiratory dysfunction, due to weakness of inspiratory and/or expiratory muscles (essential to an effective cough), lung volume changes (a combination of muscle weakness and alterations of the mechanical properties of the lungs and chest wall) and bulbar dysfunction, with loss of the ability to cough and swallow (that may result in pooling of saliva and mucus in the pharynx, predisposing to aspiration). Even so, there aren't any studies on respiratory function, quality of cough, prevalence of respiratory infections, atelectasis, acute or chronic respiratory failure, and their repercussions on morbidity and mortality in elderly people who are bedridden for a long time.
Cough augmentation techniques aim to improve cough efficiency, with potential for both short‐ and long‐term effects on pulmonary morbidity. During episodes of acute respiratory exacerbation, cough augmentation techniques could clear impacted secretions to prevent the progression to respiratory failure. In the long-term, the regular use of cough augmentation is intended to reduce the incidence and/or severity of respiratory tract infections requiring unscheduled hospitalization.
MI-E therapy is a cough augmentation technique that increases inspiratory and expiratory flow to restore cough quality and improve secretion mobilization.1 Experts suggest that using MI-E in very weak patients is a priority.2 However, the overall quality of evidence on the efficacy and safety of MI-E is very low. Most of the research on cough augmentation techniques has been carried out in chronic neuromuscular disease (NMD) and in extubation or weaning of critically-ill patients.3,4 The author couldn't find any studies on the efficacy and safety of the technique in long-term bedridden patients
The author presents a case of pulmonary atelectasis in a long-term bedridden patient, which highlights the effectiveness of a non-invasive, generally safe and relatively inexpensive technique (the MI-E), even in the context of an ED, for patients not eligible for ICU or broncoscopy.
Future research is needed to evaluate the effectiveness, potential adverse effects, precautions and contraindications of MI-E in the long-term bedridden patients. In addition to clinical outcomes, factors such as patient tolerance, adherence, treatment burden, knowledge of the technique, confidence, availability of devices, and cost should be considered by clinicians, patients, and caregivers.