Workplace exposures are widely known to cause specific occupational diseases such as silicosis and asbestosis, but they also can contribute substantially to causation of common respiratory diseases. In 2019, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) published a joint statement on the occupational burden of respiratory diseases. Our aim on this narrative review is to summarise the most recent evidence published after the ATS/ERS statement as well as to provide information on traditional occupational lung diseases that can be useful for clinicians and researchers.
ResultsNewer publications confirm the findings of the ATS/ERS statement on the role of workplace exposure in contributing to the aetiology of the respiratory diseases considered in this review (asthma, COPD, chronic bronchitis, idiopathic pulmonary fibrosis, hypersensitivity pneumonitis, infectious pneumonia). Except for COPD, chronic bronchitis and infectious pneumonia, the number of publications in the last 5 years for the other diseases is limited. For traditional occupational lung diseases such as silicosis and asbestosis, there are old as well as novel sources of exposure and their burden continues to be relevant, especially in developing countries.
ConclusionsOccupational exposure remains an important risk factor for airways and interstitial lung diseases, causing occupational lung diseases and contributing substantially in the aetiology of common respiratory diseases. This information is critical for public health professionals formulating effective preventive strategies but also for clinicians in patient care. Effective action requires shared knowledge among clinicians, researchers, public health professionals, and policy makers.
Chronic respiratory diseases, excluding lung cancer and infections, are the third leading cause of death, being responsible for 4 million deaths worldwide and 103.5 million disability-adjusted life years (DALYs), constituting 4.1 % (3.7 %–4.4 %) of global DALYs for all causes in 2019.1 The epidemiology of pneumonia, for example, highlights the importance of respiratory infections as one of the leading threats to human health.2 Thus, prevention is the keystone for reducing the impact of chronic respiratory diseases and infections on health, especially when a risk factor is avoidable, and a successful treatment is not always available.
Workplace exposures are one of the main risk factors for chronic respiratory disease-associated mortality; they are in third place after smoking and ambient particulate matter globally and rank second in some regions (Southeast Asia and Latin tropical America).1 Occupational exposures are also an important risk factor for the diffusion of respiratory infections.3
Therefore, it is crucial to set up effective prevention strategies to address the occupational burden of respiratory diseases. In 2019, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) promulgated an updated official statement on the role of occupational exposures in the aetiology of asthma and Chronic Obstructive Pulmonary Disease (COPD), as well as on quantifying the contribution of workplace exposures and other important respiratory diseases not covered by previous statements4 (including lower respiratory tract infections, idiopathic pulmonary fibrosis, hypersensitivity pneumonitis, and other diffuse parenchymal diseases).5
Furthermore, “traditional” occupational lung diseases, such as pneumoconiosis, have not disappeared. In fact, there are new workplace exposures involving old risk factors (e.g., free crystalline silica in artificial stone used as an interior building material) and the spread of dangerous production patterns in countries that may be unaware of the risk involved for workers (e.g. denim sandblasting), thereby keeping traditional occupational exposures as a matter of concern regarding their clinical and public health consequences.6 In addition, there are novel occupational lung diseases caused by emerging risk factors (e.g., Ardystil, nylon flock, indium tin oxide) that add to the occupational burden of respiratory conditions.7
For these reasons, any clinician, whether a generalist or specialist, and all public health professionals need to be aware of the importance of occupational exposures in causing or worsening respiratory conditions, given the potential to play a crucial role in early diagnosis and prevention. The aim of this brief review is to update the knowledge of clinicians and public health specialists about the occupational burden for key respiratory diseases and to provide a perspective on novel aspects of traditional occupational lung diseases.
This paper is the sixth in the Pulmonology Series on “Air pollution and health.”8–12
MethodsFor this update, we searched PubMed and Embase databases for publications appearing from the 1st of January 2018 up to 30th of June 2023, applying the same research strategy and strings used in the 2019 ATS/ERS statement,5 but with a narrative review perspective. We also reviewed reference citations in the publications identified to capture other relevant articles. Since the aim was not to produce an official update of the ATS/ERS statement, which would require a different methodological approach, we have selected some key respiratory diseases that may be of interest to clinicians, such as asthma, COPD, chronic bronchitis, idiopathic pulmonary fibrosis, hypersensitivity pneumonia and common forms of infectious pneumonia. We also have included content regarding novel aspects of more traditional occupational diseases, specifically the pneumoconioses, because of their increasing importance, especially in developing countries.
For asthma, COPD, chronic bronchitis, idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and infectious pneumonia, our analysis included publications by study type, exposure assessment, health outcomes, and specific associations derived from multivariable analysis, adjusted for other risk factors and confounders, when available. Furthermore, publications were selected similarly to what had been done in the ATS/ERS statement5: for asthma prospective longitudinal population-based studies; for COPD and chronic bronchitis population-based studies; for idiopathic pulmonary fibrosis case-control studies; for hypersensitivity pneumonitis cross sectional studies and for infectious pneumonia any kind of study. We excluded COVID-19 related-conditions because the next issue of the Series will be devoted to that disease.
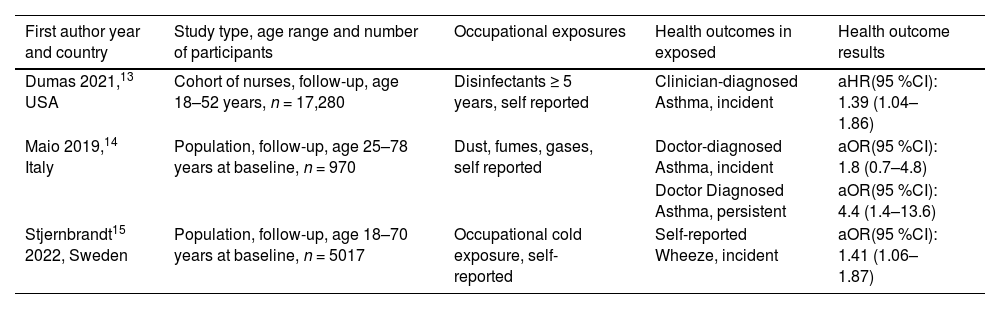
ResultsAsthmaFor asthma, although the search retrieved 3101 papers, only three13–15 reported longitudinal studies in which the incidence of asthma or asthma-associated symptoms in relation to occupation could be found as an outcome Table 1. This is a sparse publication record considering the fact that the percentage of asthma-related disability-adjusted life years (DALY) attributable to workplace exposure remains quite high and closer to the one due to smoking.16 In one of the longitudinal study the authors, during the follow-up period, found an association between occupational exposure to gas, dust and fumes and persistence of asthma, but not an increased incidence of new onset asthma.14 The same authors, in the context of a multicentre national study, suggested a possible role of lifetime occupational exposure to gas, dust and fumes in the persistence/worsening of severe asthma as well.17 Nevertheless, in individuals with occupational asthma, the prevalence of severe asthma was found to be high and often related to a persistent exposure to asthmagens at work.18 Among the new asthmagens, disinfectants may play an important role, as was highlighted by an analysis of the large American nurses’ study included in Table 1, indicating a risk associated with high exposure, even though “no” exposure to disinfectant was statistically associated with incident asthma in the same population.19 In another paper, occupational exposure to extreme cold weather was associated with an increase in the incidence of wheezing.15 In the 2018–2023 period, no systematic review was published on asthma incidence and work-related risk factors. In summary, few relevant publications were published in the study period on incident occupational asthma, even though the importance of work-related exposures regarding asthma control and the socioeconomical impact of work-related asthma is widely appreciated.18 Thus, the need remains for updated scientific evidence on the epidemiological impact of occupational exposure in asthma based on incidence data.
Longitudinal population-based and large cohort studies on occupational risk factors for asthma.
| First author year and country | Study type, age range and number of participants | Occupational exposures | Health outcomes in exposed | Health outcome results |
|---|---|---|---|---|
| Dumas 2021,13 USA | Cohort of nurses, follow-up, age 18–52 years, n = 17,280 | Disinfectants ≥ 5 years, self reported | Clinician-diagnosed Asthma, incident | aHR(95 %CI): 1.39 (1.04–1.86) |
| Maio 2019,14 Italy | Population, follow-up, age 25–78 years at baseline, n = 970 | Dust, fumes, gases, self reported | Doctor-diagnosed Asthma, incident | aOR(95 %CI): 1.8 (0.7–4.8) |
| Doctor Diagnosed Asthma, persistent | aOR(95 %CI): 4.4 (1.4–13.6) | |||
| Stjernbrandt15 2022, Sweden | Population, follow-up, age 18–70 years at baseline, n = 5017 | Occupational cold exposure, self-reported | Self-reported Wheeze, incident | aOR(95 %CI): 1.41 (1.06–1.87) |
aOR = adjusted Odds ratio; aHR = adjusted Hazard ratio.
Since the last update of the occupational burden of respiratory diseases few papers were published on asthma. There is a need of updated scientific evidence on the epidemiological impact of workplace exposure on asthma incidence.
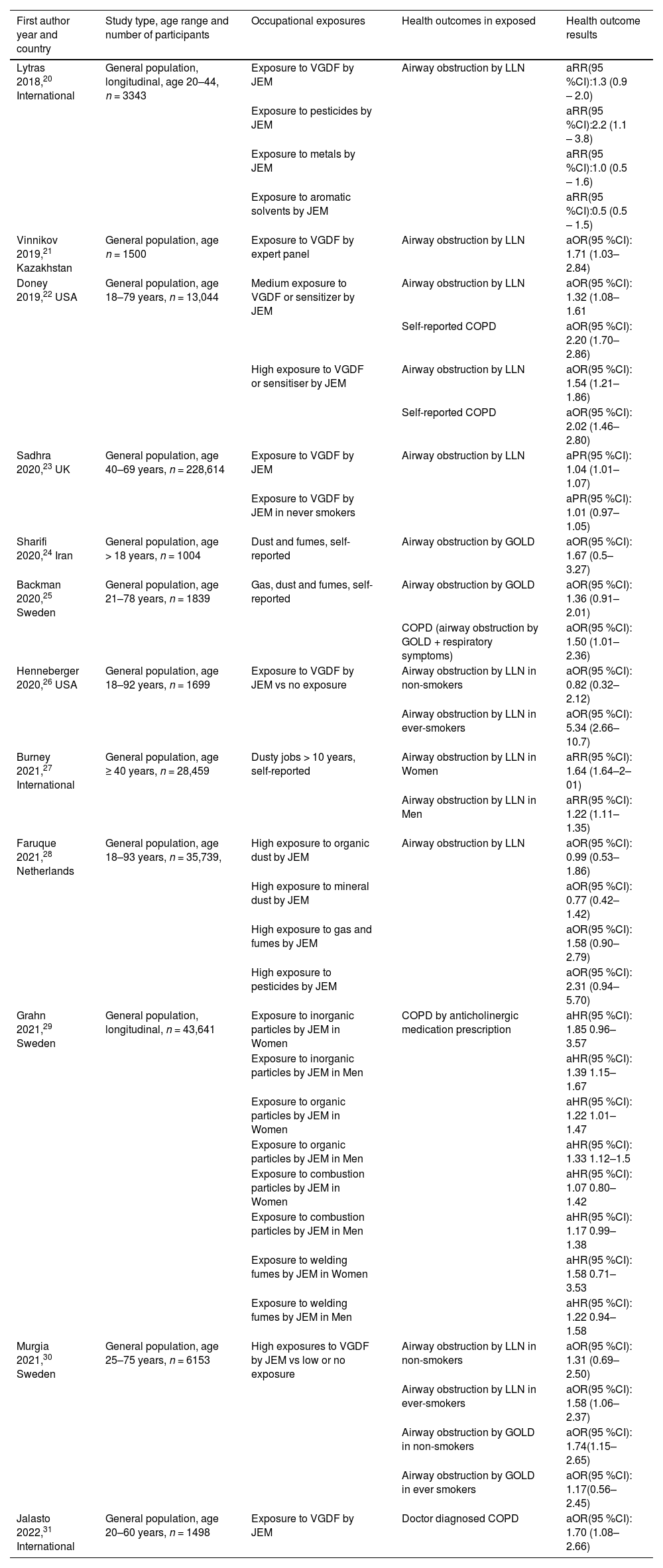
COPD and chronic bronchitisThe search retrieved 5863 documents for COPD and 2684 for chronic bronchitis. Of these, we identified 12 relevant publications for COPD20–31 and 8 for chronic bronchitis24,28,31–36Tables 2 and 3. The overall results show a slight decrease in risk-estimates compared with previous papers. This could reflect a reduction in exposure to occupational hazards in more recent years or an underestimation of the exposure. In fact, classification of exposure is a very important issue. Studies carried out in the last 5 years confirm the trend to use COPD-specific job exposure matrices instead of self-reported exposure or in-house expert opinion. However, an objective parameter, such as airway obstruction, is less influenced by the risk of a recall bias regarding exposure than a self-reported diagnosis/symptom. Another interesting aspect concerns the interaction between occupational exposure and smoking habits, which was analysed in two studies.26,30 The association between workplace exposure and airway obstruction was clear only in ever-smokers, and this could reflect an interaction between smoking and workplace pollutants, where smoking is needed to prime the inflammation in the airways to allow the noxious effects of workplace pollutants.37 Such an association highlights the relevance of occupational physicians, GP and pulmonologists advising workers exposed to vapours, gases, dusts and fumes (VGDF) or other pollutants to quit smoking. Another interesting finding is the potential role of pesticide exposure as a strong occupational risk factor for airways obstruction.20,28
Population-based studies on occupational risk factors for COPD.
| First author year and country | Study type, age range and number of participants | Occupational exposures | Health outcomes in exposed | Health outcome results |
|---|---|---|---|---|
| Lytras 2018,20 International | General population, longitudinal, age 20–44, n = 3343 | Exposure to VGDF by JEM | Airway obstruction by LLN | aRR(95 %CI):1.3 (0.9 – 2.0) |
| Exposure to pesticides by JEM | aRR(95 %CI):2.2 (1.1 – 3.8) | |||
| Exposure to metals by JEM | aRR(95 %CI):1.0 (0.5 – 1.6) | |||
| Exposure to aromatic solvents by JEM | aRR(95 %CI):0.5 (0.5 – 1.5) | |||
| Vinnikov 2019,21 Kazakhstan | General population, age n = 1500 | Exposure to VGDF by expert panel | Airway obstruction by LLN | aOR(95 %CI): 1.71 (1.03–2.84) |
| Doney 2019,22 USA | General population, age 18–79 years, n = 13,044 | Medium exposure to VGDF or sensitizer by JEM | Airway obstruction by LLN | aOR(95 %CI): 1.32 (1.08–1.61 |
| Self-reported COPD | aOR(95 %CI): 2.20 (1.70–2.86) | |||
| High exposure to VGDF or sensitiser by JEM | Airway obstruction by LLN | aOR(95 %CI): 1.54 (1.21–1.86) | ||
| Self-reported COPD | aOR(95 %CI): 2.02 (1.46–2.80) | |||
| Sadhra 2020,23 UK | General population, age 40–69 years, n = 228,614 | Exposure to VGDF by JEM | Airway obstruction by LLN | aPR(95 %CI): 1.04 (1.01–1.07) |
| Exposure to VGDF by JEM in never smokers | aPR(95 %CI): 1.01 (0.97–1.05) | |||
| Sharifi 2020,24 Iran | General population, age > 18 years, n = 1004 | Dust and fumes, self-reported | Airway obstruction by GOLD | aOR(95 %CI): 1.67 (0.5–3.27) |
| Backman 2020,25 Sweden | General population, age 21–78 years, n = 1839 | Gas, dust and fumes, self-reported | Airway obstruction by GOLD | aOR(95 %CI): 1.36 (0.91–2.01) |
| COPD (airway obstruction by GOLD + respiratory symptoms) | aOR(95 %CI): 1.50 (1.01–2.36) | |||
| Henneberger 2020,26 USA | General population, age 18–92 years, n = 1699 | Exposure to VGDF by JEM vs no exposure | Airway obstruction by LLN in non-smokers | aOR(95 %CI): 0.82 (0.32–2.12) |
| Airway obstruction by LLN in ever-smokers | aOR(95 %CI): 5.34 (2.66–10.7) | |||
| Burney 2021,27 International | General population, age ≥ 40 years, n = 28,459 | Dusty jobs > 10 years, self-reported | Airway obstruction by LLN in Women | aRR(95 %CI): 1.64 (1.64–2–01) |
| Airway obstruction by LLN in Men | aRR(95 %CI): 1.22 (1.11–1.35) | |||
| Faruque 2021,28 Netherlands | General population, age 18–93 years, n = 35,739, | High exposure to organic dust by JEM | Airway obstruction by LLN | aOR(95 %CI): 0.99 (0.53–1.86) |
| High exposure to mineral dust by JEM | aOR(95 %CI): 0.77 (0.42–1.42) | |||
| High exposure to gas and fumes by JEM | aOR(95 %CI): 1.58 (0.90–2.79) | |||
| High exposure to pesticides by JEM | aOR(95 %CI): 2.31 (0.94–5.70) | |||
| Grahn 2021,29 Sweden | General population, longitudinal, n = 43,641 | Exposure to inorganic particles by JEM in Women | COPD by anticholinergic medication prescription | aHR(95 %CI): 1.85 0.96–3.57 |
| Exposure to inorganic particles by JEM in Men | aHR(95 %CI): 1.39 1.15–1.67 | |||
| Exposure to organic particles by JEM in Women | aHR(95 %CI): 1.22 1.01–1.47 | |||
| Exposure to organic particles by JEM in Men | aHR(95 %CI): 1.33 1.12–1.5 | |||
| Exposure to combustion particles by JEM in Women | aHR(95 %CI): 1.07 0.80–1.42 | |||
| Exposure to combustion particles by JEM in Men | aHR(95 %CI): 1.17 0.99–1.38 | |||
| Exposure to welding fumes by JEM in Women | aHR(95 %CI): 1.58 0.71–3.53 | |||
| Exposure to welding fumes by JEM in Men | aHR(95 %CI): 1.22 0.94–1.58 | |||
| Murgia 2021,30 Sweden | General population, age 25–75 years, n = 6153 | High exposures to VGDF by JEM vs low or no exposure | Airway obstruction by LLN in non-smokers | aOR(95 %CI): 1.31 (0.69–2.50) |
| Airway obstruction by LLN in ever-smokers | aOR(95 %CI): 1.58 (1.06–2.37) | |||
| Airway obstruction by GOLD in non-smokers | aOR(95 %CI): 1.74(1.15–2.65) | |||
| Airway obstruction by GOLD in ever smokers | aOR(95 %CI): 1.17(0.56–2.45) | |||
| Jalasto 2022,31 International | General population, age 20–60 years, n = 1498 | Exposure to VGDF by JEM | Doctor diagnosed COPD | aOR(95 %CI): 1.70 (1.08–2.66) |
aOR = adjusted Odds ratio; aHR = adjusted Hazard ratio, aRR = adjusted Relative Risk; aPR = adjusted Prevalence ratio; VGDF = vapors, gas, dust and fumes; JEM = job exposure matrix; LLN = obstruction defined by a FEV1/FVC < lower limit if the normal; GOLD = obstruction defined by FEV1/FVC < 0.70.
Population-based studies on occupational risk factors for Chronic bronchitis.
| First author year and country | Study type, age range and number of participants | Occupational exposures | Health outcomes in exposed | Health outcome results |
|---|---|---|---|---|
| Mejza 2018,32 Poland | General population, age ≥40, n = 3558 | Pesticides, self-reported | Chronic bronchitis | aOR(95 %CI): 1.41 (1.00–2.01) |
| Chemicals, self-reported | aOR(95 %CI): 1.56 (1.15–2.12) | |||
| Asbestos, self-reported | aOR(95 %CI): 2.00 (0.68–5.84) | |||
| Lytras 2018,33 International | General population, age 20–44, n = 8794 | Exposure to VGDF by JEM | Chronic bronchitis | aRR(95 %CI): 1.14 (0.87–1.48) |
| Gonzalez-Garcia 2018,34 Colombia | General population, age 40–93 years, n = 5539 | Exposure to VGDF, self-reported | Chronic bronchitis | aOR(95 %CI): 1.44 (1.12–1.86) |
| Sharifi 2020,24 Iran | General population, age > 18 years, n = 1004 | Dust and fumes, self-reported | Chronic bronchitis | aOR(95 %CI): 2.01 (0.92-4.40) |
| Skaaby 2021,35 Denmark | General population, n = 64,279 | Low exposure to VGDF by JEM in smokers | Chronic bronchitis | aOR(95 %CI): 1.1 (1.0;1.3) |
| Low exposure to VGDF by JEM in non-smokers | aOR(95 %CI): 1.0 (0.9;1.1) | |||
| High exposure to VGDF by JEM in smokers | aOR(95 %CI): 1.3 (1.1;1.5) | |||
| High exposure to VGDF by JEM in non-smokers | aOR(95 %CI): 1.0 (0.9;1.1) | |||
| Faruque 2021,28 The Netherland | General population, age 18–93 years, n = 35,739, | High exposure to organic dust by JEM | Chronic bronchitis | aOR(95 %CI): 1.18 (0.75–1.86) |
| High exposure to mineral dust by JEM | aOR(95 %CI): 0.73 (0.46–1.15) | |||
| High exposure to gas and fumes by JEM | aOR(95 %CI): 1.45 (0.95–2.23) | |||
| High exposure to pesticides by JEM | aOR(95 %CI): 2.58 (1.32–5.07) | |||
| Jalasto 2022,31 International | General population, age 20–60 years, n = 1498 | Exposure to VGDF by JEM | Chronic cough or chronic phlegm | aOR(95 %CI): 1.75 (1.14 2.71) |
| Ratanachina 2023,36 International | General population, n = 28,823 | Exposure to organic dust by expert panel | Chronic cough | aOR(95 %CI): 1.22 (1.02–0.46) |
| Exposure to inorganic dust by expert panel | aOR(95 %CI): 1.59 (1.25–2.03) | |||
| Exposure to fumes by expert panel | aOR(95 %CI): 1.42 (1.07–1.88) | |||
| Exposure to organic dust by expert panel | Chronic phlegm | aOR(95 %CI): 1.16 (0.98–1.37) | ||
| Exposure to inorganic dust by expert panel | aOR(95 %CI): 1.40 (1.09–1.79) | |||
| Exposure to fumes by expert panel | aOR(95 %CI): 1.31 (0.98–1.75) |
aOR = adjusted Odds ratio; aHR = adjusted Hazard ratio, aRR = adjusted Relative Risk; VGDF = vapors, gas, dust and fumes; JEM = job exposure matrix;.
Yet another important aspect of the recent literature involves the definition of COPD. The most frequent definition of COPD used in epidemiological studies is (fixed) airways obstruction, even in the included studies, although one study used a definition by a pharmacological register (anticholinergic use, a treatment for COPD),29 while in another COPD was “doctor-diagnosed COPD”. Interestingly, in one study,25 the association between COPD and occupational exposure was more obvious using a more clinical definition of COPD, also comprising COPD symptoms besides the presence of airways obstruction, possibly highlighting the presence of a specific, more symptomatic phenotype of COPD, maybe more relevant to occupational exposure risk. The papers included in the Table 2 show that recent publications employ a definition of airflow obstruction based on the lower limit of the normal (LLN) of FEV1/FVC, rather than below the fixed ratio of FEV1/FVC at 0.7. Some recent studies not included in Table 2 took into account the effect of occupational exposure on the crude FEV1/FVC ratio, and in one of these studies, workplace exposure had no effect on lung function;36 this was probably influenced by the fact that the prevalence of the exposure, estimated by an expert panel, was very low compared with similar studies20 and was also lower than the self-reported exposure from the same population.27 In other studies, occupational exposure was associated with a reduction in FEV1/FVC ratio, though not with excess lung function decline in a follow-up period of 4.5 years.38 In addition to the new studies summarized above, multiple reviews of occupational exposure and COPD have been published,39–41 confirming the 2019 ATS/ERS statement findings with an average increased odd of COPD related to occupational exposure ranging between 40 % and 69 % (OR 1.40–1.69).
The same assumptions made for COPD are valid for chronic bronchitis Table 3: for example, the emerging role of pesticide exposure on chronic bronchitis symptoms. Some of the studies24,32,34 used self-reported exposure to occupational pollutants (e.g. dusts, fumes, etc.) as an exposure indicator: in fact, this can be at higher risk of recall bias in subjects with chronic bronchitis symptoms than in healthy subjects. Again, in one study, chronic bronchitis was associated with occupational exposures only in ever smokers, suggesting an interaction between these risk factors in producing airway inflammation and respiratory symptoms such as cough and phlegm.33 Finally, studies with imaging features of COPD and occupational exposure are increasing. In one of these studies, exposure to VGDF was associated with lung CT-scan abnormalities, reflecting a real pulmonary anatomical impairment rather than just symptoms of airflow limitation.42
There is growing evidence of the impact of workplace exposure to vapours, gases, dusts and fumes on COPD and chronic bronchitis occurrence, also in developing countries
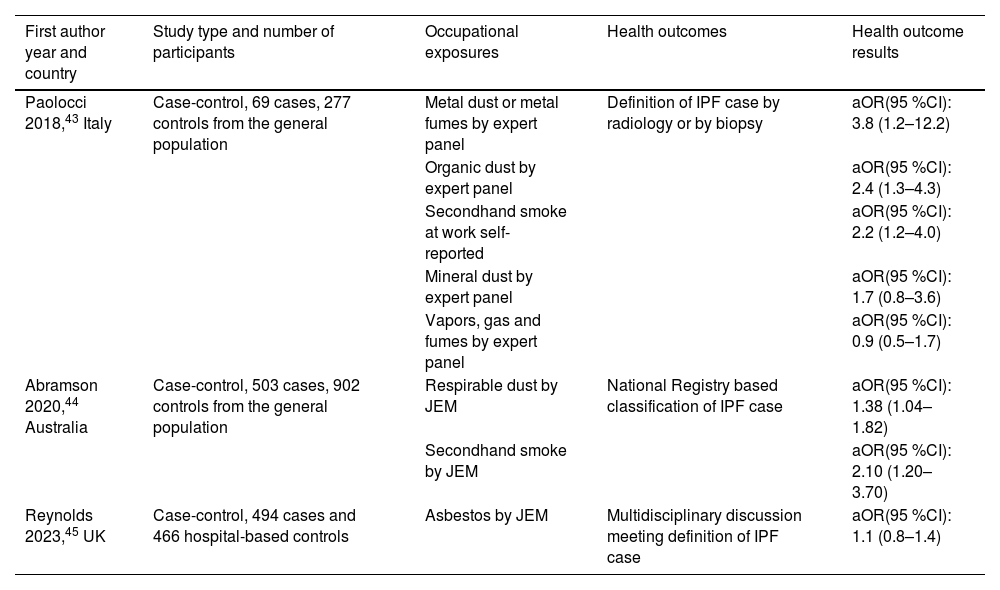
Idiopathic pulmonary fibrosis (IPF) and hypersensitivity pneumonitisThe search retrieved 515 articles on the association between occupational exposure and IPF, among which three were case-control studies, meeting our inclusion criteria43–45Table 4. In addition, there was an equal number of newly published systematic reviews on this topic.46–48 This may reflect a growing interest in more definitive diagnostic criteria in light of emerging therapeutic options for IPF.49 The latest evidence confirms the association between dust or second-hand cigarette smoke exposure and IPF. In contrast, asbestos exposure does not seem to be associated with the occurrence of this disease. However, one of the three studies suggested an interaction between asbestos and smoking in increasing the risk of IPF, especially among those having a specific genotype48 and another showed an increased risk of IPF in those who were highly exposed to asbestos.47 Two studies included general population controls, whilst the other considered hospital-based referents. The study carried out in Australia has some characteristics that distinguish it from the other two studies: in particular the sample size was larger, and a specific job exposure matrix (JEM) was used. Interestingly, smoking was not a risk factor in one study46 and it needed the co-exposure with asbestos to be a risk factor in another study.48 Conversely, second-hand cigarette smoke was associated with the occurrence of IPF in all three studies. Finally, duration of exposure to risk factors played an important role strengthening the association between these work-related risk factors and IPF.46
Case-control studies on occupational risk factors for idiopathic pulmonary fibrosis.
| First author year and country | Study type and number of participants | Occupational exposures | Health outcomes | Health outcome results |
|---|---|---|---|---|
| Paolocci 2018,43 Italy | Case-control, 69 cases, 277 controls from the general population | Metal dust or metal fumes by expert panel | Definition of IPF case by radiology or by biopsy | aOR(95 %CI): 3.8 (1.2–12.2) |
| Organic dust by expert panel | aOR(95 %CI): 2.4 (1.3–4.3) | |||
| Secondhand smoke at work self-reported | aOR(95 %CI): 2.2 (1.2–4.0) | |||
| Mineral dust by expert panel | aOR(95 %CI): 1.7 (0.8–3.6) | |||
| Vapors, gas and fumes by expert panel | aOR(95 %CI): 0.9 (0.5–1.7) | |||
| Abramson 2020,44 Australia | Case-control, 503 cases, 902 controls from the general population | Respirable dust by JEM | National Registry based classification of IPF case | aOR(95 %CI): 1.38 (1.04–1.82) |
| Secondhand smoke by JEM | aOR(95 %CI): 2.10 (1.20–3.70) | |||
| Reynolds 2023,45 UK | Case-control, 494 cases and 466 hospital-based controls | Asbestos by JEM | Multidisciplinary discussion meeting definition of IPF case | aOR(95 %CI): 1.1 (0.8–1.4) |
aOR = adjusted Odds ratio; JEM = job exposure matrix.
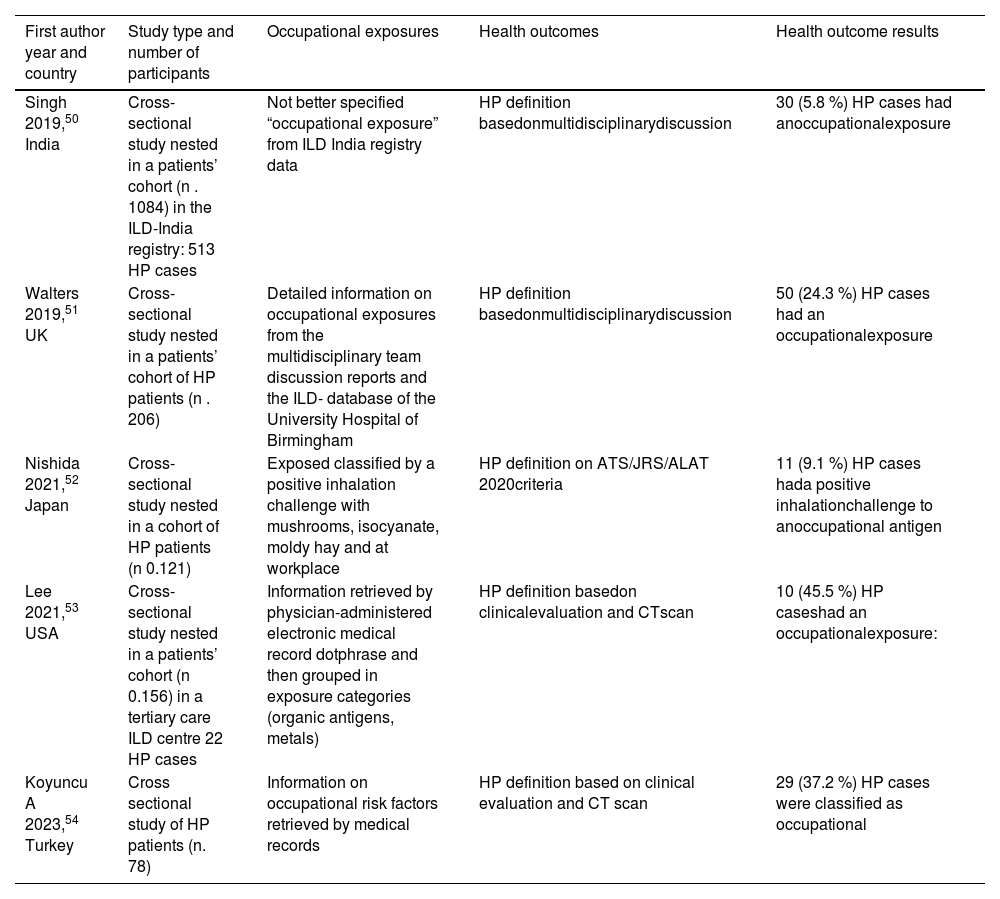
For hypersensitivity pneumonitis (HP) the search identified 252 articles from which five references were cross-sectional studies.50–54Table 5 The prevalence of occupational exposure ranged between 5.8 % and 45.5 %. All the studies were cross-sectional or case-series of HP patients or based on national or local databases of interstitial lung diseases. The exposure was retrieved mainly from information in patient records. In one study52 the classification was based on a positive specific inhalation challenge with occupational antigens. The diagnosis of HP was based on multidisciplinary panel discussion or on single institution's experience. The results were consistent with the findings of the 2019 ATS/ERS statement. In one study, the prevalence of occupational exposure was rather low,50 which may reflect a specific local pattern of exposure, where environmental exposure could be more frequent, as was already reported in the ATS/ERS statement.5 In another study53 the cases attributed to the occupational exposure were only 9.1 %, but in this instance the attribution was made by a positive specific inhalation challenge, which makes it difficult to compare these results with the others. Interestingly, in the time frame considered, a single systematic review on occupational causes of HP was published,55 which included a detailed list of occupational agents that have been associated with HP, in the supplementary material.
Studies on the prevalence of work related hypersensitivity pneumonitis (HP) in cross-sectional studies.
| First author year and country | Study type and number of participants | Occupational exposures | Health outcomes | Health outcome results |
|---|---|---|---|---|
| Singh 2019,50 India | Cross-sectional study nested in a patients’ cohort (n . 1084) in the ILD-India registry: 513 HP cases | Not better specified “occupational exposure” from ILD India registry data | HP definition basedonmultidisciplinarydiscussion | 30 (5.8 %) HP cases had anoccupationalexposure |
| Walters 2019,51 UK | Cross-sectional study nested in a patients’ cohort of HP patients (n . 206) | Detailed information on occupational exposures from the multidisciplinary team discussion reports and the ILD- database of the University Hospital of Birmingham | HP definition basedonmultidisciplinarydiscussion | 50 (24.3 %) HP cases had an occupationalexposure |
| Nishida 2021,52 Japan | Cross-sectional study nested in a cohort of HP patients (n 0.121) | Exposed classified by a positive inhalation challenge with mushrooms, isocyanate, moldy hay and at workplace | HP definition on ATS/JRS/ALAT 2020criteria | 11 (9.1 %) HP cases hada positive inhalationchallenge to anoccupational antigen |
| Lee 2021,53 USA | Cross-sectional study nested in a patients’ cohort (n 0.156) in a tertiary care ILD centre 22 HP cases | Information retrieved by physician-administered electronic medical record dotphrase and then grouped in exposure categories (organic antigens, metals) | HP definition basedon clinicalevaluation and CTscan | 10 (45.5 %) HP caseshad an occupationalexposure: |
| Koyuncu A 2023,54 Turkey | Cross sectional study of HP patients (n. 78) | Information on occupational risk factors retrieved by medical records | HP definition based on clinical evaluation and CT scan | 29 (37.2 %) HP cases were classified as occupational |
The latest scientific evidence confirms an association between occupational exposure to airborne pollutants and IPF or HP, consistent with the findings of the 2019 ATS/ERS statement.
PneumoniaA previous ATS/ERS statement summarised data showing that welders and other workers exposed to metal fumes and inorganic dusts have an increased risk of pneumococcal pneumonia, with the attributable fraction for welders exceeding 50 %5. In the general population, the population attributable fraction for community acquired pneumonia (CAP) was 10 %. We combined a literature search with a narrative approach including original papers, case reports and reviews published in English. Of the 1234 papers retrieved, we identified 28 original papers and three additional papers from the reference lists. Of the 28 original papers, 22 were excluded because of lack of relevant outcome, ambient air pollution, or predictors for vaccinations. The eight publications retained were about pneumococcal pneumonia,56–59 welder's anthrax,60–61 Legionella,62 and Coccidiomycosis.63 We also identified three relevant reviews,64–66 and three case reports.57,67–69
A population-based case-control study of pneumococcal pneumonia found that occupational exposures to inorganic dust, especially silica dust and fumes, including metal fumes, in the year preceding the pneumonia episode increased the disease risk.56 Cumulative exposure further increased the odds for pneumococcal pneumonia.58 Of note, these associations had already been reported in the 1920s.65 Further, working in close contact with other workers, as well as outside work, also increased the risk of pneumococcal pneumonia.59 Clusters of pneumococcal pneumonia cases have been reported in shipyards and construction sites. For example, thirty cases of pneumococcal pneumonia were reported in a shipyard in Finland.67 Most of the cases worked in the docks as plumbers, electricians, welders, or supervisors. Another outbreak involved 20 cases of pneumococcal pneumonia that were reported in a Norwegian shipyard, and most of the patients worked with welding and interior outfitting.68 An outbreak of pneumococcal pneumonia was also detected in a French shipyard.69 The working conditions were described as crowded, with 102 different nationalities and occupational exposures to irritants, metal fumes, dust and chemicals. A subsequent genomic sequencing of outbreak isolates from non-sterile specimen (e.g. nasopharyngeal swab) showed that the Finnish and Norwegian outbreaks were similar, with a common ancestor dated from around 2017.57 Some relevant pathogenetic mechanisms for the increased risk of pneumococcal infections in relation to dust exposure may involve: inorganic dust reducing ciliary beating and initiating overproduction of mucus, which also impairs mucociliary clearance; welding fumes upregulating expression of platelet-activating factor receptor (PAFR) which enhances pneumococcal adherence to epithelial cells; dust particles aiding nutrient acquisition for pneumococci by increasing the permeability of the epithelium thereby augmenting the influx of glucose; and by being filled with dust particles, alveolar macrophages reducing their phagocytosis of pneumococci(possibly the most important mechanism).66
Legionella infections have been described among hotel workers, civil engineering labourers and professional drivers.64 One paper from Japan described three workers who developed Legionella pneumonia when engaged in reconstruction after a period with very heavy rainfall.62 Exposure to infected soil was mentioned as a possible cause of this outbreak.
Seven workers were reported to have an infection caused by an anthrax toxin-expressing Bacillus cereus, and the clinical picture was pneumonia.61 Six of them were welders and one was a metal-worker. Exposure to infected soil was also mentioned as a possible explanation, as most of the cases worked outdoors.
Coccidiomycosis, also known as Valley fever, is caused by inhalation of spores of the fungus Coccidiodes spp, which grows in soil of semi-arid areas.63 Most affected individuals are asymptomatic, but 30–40 % develop fever and a pneumonia-like clinical picture. A cluster of Coccidiomycosis was described among workers constructing solar farms in California.63 Exposures originated from soil-disruptive work, digging and working in trenches.
In conclusion, recent data continue to identify occupational risk factors as potentially important in the prevention of community-acquired pneumonia. The first line of prevention should be reduction of workplace exposures to vapours, gas, dust and fumes. However, despite regulatory activities exposure to dust and fumes may still occur. In such circumstances, it is necessary to provide additional protection by personal respiratory protection. Regarding pneumococcal pneumonia, pneumococcal vaccination should be considered, especially for workers in crowded conditions or with exposure to metal fumes or inorganic dust.
The exposure to occupational pollutants may have an important role in predisposing to infectious pneumonia. For some risk factor (welding fumes) this association is consistent.
PneumoconiosisPneumoconiosis, a range of respiratory conditions resulting from occupational inhalation of mineral dusts, remains a persistent global health concern. Inadequate measures to prevent dust exposure, delayed diagnosis, and limited availability of effective treatments contribute to this problem.70 The impact of pneumoconiosis on global health is evident, with substantial new cases and DALYs reported in 2019, particularly in China, where the burden of DALYs attributable to pneumoconiosis is disproportionately high.71,72 While the age-standardised incidence (ASIR) and DALY rates associated with pneumoconiosis have seen a gradual decrease between 1990 and 2019, a notable disparity between genders still remains, with a larger increase in incidence observed in males above the age of 20 years.73,74 There has been an upward trend in ASIRs of Asbestosis, particularly in high-income regions like North America and Australasia.75 These findings highlight the ongoing challenges and regional variations in addressing pneumoconiosis as a global health issue.
SilicosisIn recent years, there have been notable outbreaks of silicosis in industries not traditionally associated with silica exposure, specifically sandblasted jeans production and the fabrication of artificial kitchen and bathroom countertops from solid surface composites and engineered stone.71,76 While the former has been predominantly reported from Turkey,77 the latter has impacted various countries, including Italy, Israel, Australia, Spain, the United States, China, and Belgium.78–85 Former jeans sandblasters have been shown to develop silicosis consistently over time,86 with the severity of radiological findings and respiratory function decline at the time of diagnosis closely associated with both mortality and premature death.87 Premature death additionally has been linked to sandblasting Teflon pans and tuberculosis.88 The findings of the multinational registry, representing a distinct effort to compare demographic, exposure, and clinical data among silicosis-affected engineered stone workers, indicate a considerable and growing global population afflicted with severe and irreversible silica-associated diseases.89 Silicosis persists in previously identified areas, including sandstone mining90 and stone crushing.91 Artificial stone-associated silicosis, in contrast with natural stone-related cases, exhibits a shorter latency period, rapid radiological progression, accelerated decline in lung function, and elevated mortality.92 Silicosis, resulting from prolonged exposure to silica, is correlated with an elevated risk of tuberculosis and impaired immune function, with a notable association observed among artificial stone benchtop fabricators.93,94 Asthma prevalence could potentially be highest among workers engaged in the manufacturing of artificial stone material, particularly those exposed to phthalic anhydride and epoxy resins.79 The occurrence of connective tissue disease is prevalent among females and in individuals of both sexes with advanced stages of pneumoconiosis.95 A comprehensive and globally coordinated response is essential to mitigate the significant impact of silica-associated diseases.96 Emphasizing the utmost importance of primary prevention becomes evident, as relying solely on secondary prevention measures proves inadequate in effectively mitigating the extensive consequences of these diseases.97
Coal workers’ pneumoconiosis (CWP)The global prevalence rates of Coal workers’ pneumoconiosis (CWP) across various periods and regions demonstrated a decline from the pre-1970 period to 1981–1990. However, it experienced a subsequent increase during 1991–2000 before eventually reaching a low value of 2.29 % in 2011–2020.98 Over the period from 1990 to 2019, there was a notable decrease in the incident cases of CWP on a global scale.99 Over the past three decades, Europe, China, and the US have consistently exhibited the highest rates of CWP prevalence.98 In the US, there has been a concerning rise in pneumoconiosis prevalence among underground coal miners, particularly in the central Appalachian region.100 This increase is accompanied by a notable upsurge in progressive massive fibrosis,101 reversing the previous decline attributed to implementation and enforcement of the Coal Act.102 Despite growing interest in renewable energy, such as solar and wind power, coal remains a primary fuel for electricity generation and steel manufacture internationally. Coal mine dust lung disease (CMDLD), including conditions like coal worker pneumoconiosis (CWP), silicosis, obstructive lung disease, and dust-related diffuse fibrosis (DDF), remains a relevant health concern globally.103 The composition of respirable dust in underground coal mines extends beyond pure coal, encompassing particles derived from cutting roof and floor rock, diesel exhaust emissions from equipment, and rock dusting. A systematic review identified multiple contributing factors to the escalation of lung diseases, including mine type, geographic location, technological advancements, automation levels, thin coal seam mining, implementation of rock dusting, coal rank, and shifts in mining practices.104 Notably, mining practices involving digging for thinner and harder-to-reach seams increase rock cutting, leading to higher silica exposure. The econometrics analysis found strong evidence of increased CWP risk among coal workers in underground mines compared with surface operations. Workers in smaller mines were particularly vulnerable to CWP, as were those involved in thin-seam underground mining.105 A high prevalence of latent tuberculosis (TB) infection has been observed among individuals with CWP in China.106 Furthermore, the stage of CWP, poor workplace ventilation, family history of TB, and exposure to TB have been identified as independent risk factors for the development of active pulmonary TB in CWP patients.107
AsbestosisThe global burden of asbestos-related diseases is increasing, particularly among older men in countries like Brazil, China, Kazakhstan, and Russia, which are major asbestos producers. China, in particular, is facing a rising burden of asbestos-related disease, primarily affecting men,108 especially in rural areas, possibly due to unhealthy and unsafe working conditions.109 The occupations with the highest rates of illness due to asbestos exposure were general asbestos workers (40 %), miners (22 %), and textile workers (9 %), followed by naval, automotive, carpentry, doll-making, construction, upholstery workers, as well as those involved in the rescue, recovery, cleaning, and restoration of the World Trade Centre (4 %).110 Clinical research comprises the largest proportion (65.0 %) of research on asbestos, followed by laboratory (26.5 %) and public health (24.9 %) areas. Public health research has shown a faster decline (−5.7 % per year). Variations exist among the top 11 countries, with Finland and Italy prioritizing public health, while China and the Netherlands have lower emphasis.111 While many countries worldwide have imposed bans or restrictions on asbestos, it is noteworthy that certain countries, including Russia, China, Brazil, India, and Indonesia, continue to be significant producers and users of this hazardous material.112 However, it is important to note that despite these measures, the legacy of past asbestos use continues to pose risks.75 Asbestos-containing materials may still be present in older buildings and infrastructures, necessitating proper management and remediation to protect public health. In both Australia113 and Korea,114 despite a long-standing asbestos ban and the cessation of asbestos use respectively, a concerning trend emerges: Australia has recently experienced a peak in asbestos-related diseases, while Korea faces a rise in such cases. The decline in hospital resource utilisation among patients diagnosed with asbestosis or silicosis in Italy between 2001 and 2018 is attributed to the implementation of occupational health policies in the 1990s, aimed at mitigating exposures to asbestos and silica.115 From 1999 to 2018, asbestosis, primarily linked to the construction industry, ranked as the most frequently reported pneumoconiosis in the US.116 Notably, sheet metal workers in the US who started their careers after the implementation of environmental and occupational regulations experienced significantly lower rates of asbestos-related diseases.117 In asbestos textile workers employed from 1946 to 1984, longer exposure duration was associated with an increased risk of asbestosis death (HR 2.4 for ≥15 years vs. <5 years, p = 0.014), while a longer time since last employment was linked to a decreased risk (HR 0.3 for ≥25 years vs. <5 years, p = 0.004). Notably, individuals exposed after 1968 experienced a significant decline in the risk of asbestosis mortality.118 Effective global implementation of an asbestos ban is essential to combat asbestosis. Although some countries have achieved a decline in asbestosis incidence after banning asbestos, it remains a persistent issue due to the disease's long latency period and eventually with the occupational exposure related to the removal of asbestos. Furthermore, the global challenge continues due to dense populations in countries that still produce and use asbestos.
Silicosis and asbestosis are sometimes considered old diseases; conversely, they are increasing again from exposure in countries where dangerous productions were moved to and in new occupational settings, also in western countries.
DiscussionIn this review the scientific evidence of the occupational burden of some important respiratory diseases has been updated since the last ATS/ERS statement.5 Pulmonologists and other readers should read it to find useful data, which would help them in clinical practice to know whether an occupational exposure has or had a role in determining or in worsening the respiratory disease of their patient.
This updated narrative review of the current evidence on the occupational burden of respiratory disease offers some interesting discussion points.
First, workplace exposures remain an important risk factor for respiratory diseases, despite a general improvement in preventive strategy.
Preventive measures and globalisation are probably responsible for the slight improvement in risk estimates that we are now experiencing when analysing the results of the large population-based studies performed in the last 40 years in Western countries. However, the occupational burden is still high and pushes the entire society towards providing further improvement in terms of prevention. Moreover, some of the more encouraging studies36 may have a relevant bias in exposure assessment, making it difficult to draw definitive conclusions. In general, exposure assessment has improved by moving from subjective evaluations or self-reported exposure to analysis of more objective aspects, such as job exposure matrices, often integrated by information from structured questionnaires. Nevertheless, JEMs have some limitations to overcome, they may be valid at a single country level119 but extension to other countries can be difficult. Furthermore, they usually have some problems in following-up the exposure assessment over a long period, as is requested in long lasting longitudinal studies.
Focusing on asthma incidence and workplace, we have seen a progressive reduction in longitudinal studies during the last 20 years. This may depend on many factors, for example a general reduction in population-based studies designed for asthma in recent years due to a lack of resources for this type of survey. However, the opportunities given by big data analysis, pooled analysis, linkage to registry data could help researchers to have useful material to study whether the occupational burden of asthma is declining or not.
In the last 20 years the interest about occupational exposure and COPD/Chronic bronchitis has progressively increased and the results of this update confirm this trend. The relationship between smoking and work-related risk factors in causing airway obstruction is intriguing. This will reinforce the importance of workplace health promotion towards a total worker health approach, where primary prevention measures to avoid or reduce the exposure are linked to health promotion intervention, in this specific case, to smoking cessation.
Another relevant finding to take into account as a risk factor for COPD and chronic bronchitis concerns the observed increased risk associated with pesticide use, which could have important consequences at the general population level. Even in this case, the workplace could act as a unique laboratory to provide preventive solution applicable also to those who are non-occupationally exposed.
Research on the association between occupational exposure and COPD and chronic bronchitis needs to be improved by using better exposure assessment and the development of new diagnostic methods to intercept the disease before it becomes non-reversible, especially in occupational settings. Unfortunately, no method is available yet, and the use of imaging with CT scan, even if interesting to understand the pathogenesis of the disease, may generate some concern in terms of radioprotection, especially when imaging is used for screenings.
However, one important component of chronic obstructive diseases is emphysema, which is defined anatomically as destruction of parenchyma and loss of alveolar walls. Emphysema can be suggested by spirometry (increased residual volume and reduced diffusing capacity) but is usually diagnosed by high-resolution computed tomography (HRCT) of the lungs. COPD and emphysema are overlapping conditions, but emphysema may exist without airflow limitation and 50 % of subjects with COPD do not have emphysema.120 In recent years, studies have been performed with HRCT to examine the relation between occupational exposures and emphysema in COPD patients 121,122 and in the general population,123 finding an association between VGDF exposure and emphysema. Technological improvement in imaging could expand these kinds of studies, overcoming radioprotection and economic sustainability issues.
The interest generated by a better definition of IPF and therapeutic options for patients with IPF and progressive pulmonary fibrosis have not yet reached the field of prevention. The number of available case-control studies on the association between occupational exposures and fibrosis is limited. The most recent papers have confirmed the previous results for dust and second-hand smoke, but the role of asbestos seems less relevant, maybe reflecting a better disease definition after the introduction of new guidelines, which would avoid classifying an asbestosis as IPF.
The recent literature on hypersensitivity pneumonitis is confirmatory in regard to the ATS/ERS statement, with about one third of the cases having been classified as occupational, thereby enhancing the importance of workplace antigen avoidance as an effective preventive strategy. However, in these studies the criteria to assess the role of the occupational exposure are different, and there is a lack of case-control studies in cases in which the aetiology is unclear.
Work-related respiratory tract infections other than COVID-19 are a relatively new field and the lack of articles published in the last five years is not surprising, considering also recent pandemic. However, the available evidence supports the role of mineral dust and fumes as predisposing factors for pneumococcal severe infections and the role of mineral dust, especially silica, in interacting with the immunological system.124
Finally, an important issue is the upsurge of traditional occupational lung diseases, such as silicosis, in new occupational settings, as demonstrated by the experience with engineered stones and denim sandblasting. For this reason, clinicians should also be prepared to see diseases in their clinical practice which were wrongly considered to have almost disappeared.
ConclusionsOccupational exposures are still an important risk factor for airways and lung diseases, and this update produced 5 years after the ATS/ERS statement on the occupational burden of respiratory diseases, confirms the results with some interesting new findings regarding COPD and chronic bronchitis.
Scientific evidence derived from retrieved epidemiological studies is crucial for clinicians, in particular for pulmonologists, to understand the importance of preventing noxious occupational exposures. In fact, respiratory diseases are often chronic and irreversible, making prevention the best option for anyone. Understanding the importance of occupational exposures could also help pulmonologists or other clinicians to manage their patients in clinical practice, specifically in terms of avoiding persistent exposure that could worsen respiratory disease. For these reasons, it is always important to gather information from the patients about their job and their workplace as well as, if necessary, seeking advice from a specialist in occupational medicine who could help improve etiological diagnosis and provide the best preventive strategy.
The authors have no conflicts of interest to declare.