Several forms of pulmonary disease can occur among patients treated with amiodarone (usually delivered as an antiarrhythmic drug). Computed tomography findings indicative of amiodarone-induced lung disease include high-attenuation parenchymal-pleural lesions and nonspecific pulmonary infiltrates. Only a few cases in the literature have described the occurrence of amiodarone-induced pulmonary disease as pulmonary nodules. The authors describe a patient showing bilateral, peripheral, predominantly basal ground-glass and reticular opacities consistent with a non-specific interstitial pneumonia (NSIP) radiological pattern. This was followed by the occurrence of two nodules that progressively decreased in size after oral steroids had been given and therefore they were interpreted as an unusual manifestation of amiodarone-related pulmonary toxicity (APT).
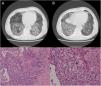
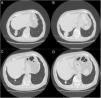
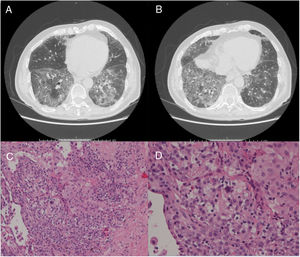
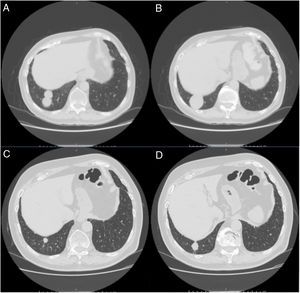
A 79-year-old man, a non-smoker and who had a history of atrial fibrillation, was treated with amiodarone 400mg daily for 3 years. During the last 2 months of treatment, he was presented with exertional dyspnea and dry cough. Respiratory function tests revealed a restrictive ventilatory pattern with a moderate reduction in carbon dioxide lung diffusion (DLCO) (14.3mL/min/mmHg, 55% of predicted value). Chest computed tomography (CT) showed bilateral, peripheral, predominantly basal ground-glass and reticular opacities consistent with a NSIP radiological pattern (Fig. 1, panels A and B). The bronchoalveolar lavage showed a significant amount of foamy macrophages. Transbronchial lung biopsy of the right lower lobe was performed and the histological examination revealed the presence of septal widening with type II pneumocytes hyperplasia, areas of organized interstitial fibrosis with sporadic fibrinous exudates, fibroblasts and collagen deposition next to aggregates of inflammatory cells and considerable amount of foamy histiocytes. These findings were consistent with a diagnosis of APT (Fig. 1, panels C and D). Other etiologies of interstitial lung disease (ILD) were carefully ruled out. Amiodarone was suspended while prednisone 40mg daily and oral anticoagulants were given, with rapid clinical and functional recovery. At 40 days, ground-glass and reticular opacities had almost completely resolved on CT scan while two soft-tissue nodules of 25 and 11mm, respectively, were identified in the right costophrenic sulcus (Fig. 2, panels A and B). Both lesions presented elevated density on CT scan images with Hounsfield Unit (HU) values ranging from 46 to 50. The patient underwent a new bronchoscopy with bronchoalveolar lavage of the right lower lobe, but microbiological and cytological investigations were unremarkable. A tuberculin skin test and blood serological markers for autoimmunity, inflammatory and infectious disease were also performed with negative results. Moreover a supplemental investigation was conducted excluding the onset of new drug treatment, trauma or exposure to environmental agents. Given their rapid onset, the elevated HU values and the exclusion of other coherent etiologies, the nodules were interpreted as an unusual subacute manifestation of APT. As physical insult to pulmonary parenchyma is known to increase susceptibility to toxicity even if low dose amiodarone treatment is used,1 surgical lung biopsy was not performed and radiological follow-up was started. At 18 months, follow-up CT showed a considerable reduction in the size of both lung nodules (Fig. 2, panels C and D) and the clinical condition of the patient was unremarkable.
(Panels A and B) CT scan images showing predominantly basal, bilateral, peripheral ground-glass opacities with associated reticular abnormalities consistent with a non-specific interstitial pneumonia (NSIP) radiological pattern. (Panels C and D) Histological appearance at different magnifications of transbronchial biopsies showing areas of organized fibrosis with fibrinous exudates and significant amount of inflammatory cells and with no evidence of malignant cells.
(Panels A and B) CT scan images showing the resolution of the ground-glass and reticular opacities after amiodarone withdrawal and the onset of two large nodules of 25mm and 11mm, respectively, which ensued in the right costophrenic sulcus. (Panels C and D) Follow-up CT images showing a substantial reduction in size of the right costophrenic nodules 18 months after amiodarone withdrawal.
Amiodarone, one of the most widely used antiarrhythmic agents, is known to cause adverse lung effects in approximately 5% of treated patients.2 Several risk factors for the development of lung complications have been identified: the pre-existence of lung disease and/or respiratory failure requiring high oxygen mixtures, lower respiratory tract infections, older age, treatment duration and a history of cardiothoracic surgery.3 Since the patient had been taking amiodarone for 2 years before developing respiratory symptoms, a dose accumulation effect might be suspected in this case.2,3 For most patients, the diagnosis of amiodarone-induced pneumopathy relies on imaging.4 According to available literature, lung involvement presents a wide range of possible manifestations: from asymptomatic lipoid pneumonia, which is usually named the ‘amiodarone effect’, to the ‘amiodarone toxicity’ spectrum, which embraces different clinical entities such as eosinophilic pneumonia, chronic organizing pneumonia (COP), acute fibrinous organizing pneumonia (AFOP), nonspecific interstitial pneumonia (NSIP)-like and idiopathic pulmonary fibrosis (IPF)-like interstitial pneumonia, desquamative interstitial pneumonia (DIP), acute respiratory distress syndrome (ARDS), diffuse alveolar hemorrhage and, more rarely, isolated or multiple nodular or mass-like lesions.2,4,5 Amiodarone-related pulmonary nodules usually generate high attenuation areas on CT scans due to the incorporation of iodine-rich amiodarone into type II pneumocytes.6 In this form, the radiological presentation of drug-induced toxicity might mimic malignant neoplasms.7 In most cases, patients respond well to the withdrawal of amiodarone with the addition of corticosteroid treatment, with symptoms and radiological abnormalities resolving within several months due to the long half-life of the amiodarone metabolites.8
The peculiarity of our case was the sequential occurrence of reversible interstitial NSIP-like lung abnormalities followed by nodular high-density opacities. Moreover, the onset of lung nodules occurred after the withdrawal of amiodarone and once corticosteroid treatment had been started. Several hypotheses on the late-onset of lung nodules could be made. A late-onset direct toxic injury to lung parenchyma and/or a slow immunologic reaction should not be excluded.2 However given that the nodules were located in the same lobe where the first biopsy had been performed, a possible increase in lung susceptibility to amiodarone toxic effect after physical insult might be suspected.1
To the best of our knowledge, this is the first case of a biphasic manifestation of amiodarone-related lung toxicity with large reversible nodules following interstitial abnormalities. The broad imaging manifestations of APT may account for some temporal heterogeneity.
Consent to publish dataInformed consent to publish data was obtained by the patient.
FundingThe authors declare that no funding was received for this paper.
Conflicts of interestThe authors have no conflicts of interest to declare.
We want to thanks Professional Editor Colin Woodham for language editing.