Lung congenital malformations, which include congenital cystic adenomatous malformation (CCAM) and pulmonary sequestration (PS), are rare. PS accounts for 0.15–6.45% of lung malformations and is characterized by a nonfunctioning mass of pulmonary tissue that receives arterial supply from the systemic circulation and lacks communication with the tracheobronchial tree. It is classified as intralobar PS (IL-PS) if it is contained within the normal lung and drains to the pulmonary veins and as extralobar PS (EL-PS) if it is separated from the surrounding parenchyma by his own pleural covering and drains to the systemic circulation.1–3 CCAM is responsible for 25–30% of congenital lung malformations. It is a hamartomatous lesion with anomalous development, characterized by a mass of lung tissue from different pulmonary origins and various degrees of cyst formation that communicates with the tracheobronchial tree and is supplied and drained by the pulmonary circulation.1–3 CCAM is classified into 5 types, based on histological features and level of malformation in the airway and lung.4 These lesions are usually diagnosed in the neonatal period, their presentation in adulthood is less frequent.1,2 Despite the fact that they are two of the most common lung malformations their association is uncommon, particularly for IL-PS and CCAM.2,5
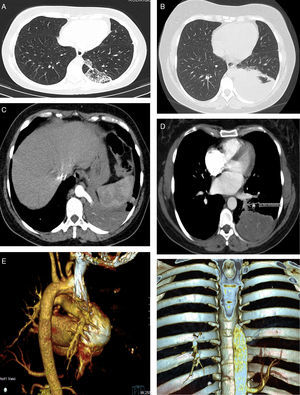
We report a case of a 36 year-old woman, never smoker, observed in the outpatient clinic for recurrent pneumonias. Over the past 5 years, she reported several pneumonias, all slowly resolving and in need of multiple courses of antibiotics. Between infections she complained of dry cough and left pleuritic chest pain. She worked as a care assistant in a continuing care unit and apart from the recurrent infections had no other significant medical history. Physical examination was unremarkable and laboratory findings and bronchoscopy were normal. Chest X-ray showed a reticular opacity in the left lower field and a computed tomography (CT), performed two months earlier, showed a heterogeneous consolidation on the left lower field with millimetric air spaces compatible with cysts (Fig. 1A). Chest CT was repeated, revealing a 10cm heterogeneous consolidation on the left inferior lobe, with arterial supply arising from the thoracic descending aorta and venous drainage to the pulmonary veins (Fig. 1B–F). The second CT was performed during another infectious episode and radiologic findings suggested IL-PS with superimposed infection. The infection was resolved and the patient was submitted to left inferior lobectomy with an uneventful post-operative recovery. Surgical procedure confirmed the anomalous vascularization and histological findings were compatible with CCAM type 2. A diagnosis of CCAM type 2 associated with IL-PS was established based on imaging and histological findings. The patient remained asymptomatic 1 year after surgery.
(A) The first chest CT performed, showing a heterogeneous posteromedial consolidation in the left lower lobe comprising multiple cysts. (B) The second chest CT showing posteromedial hepatization of the left lower field, compatible with superimposed infection. (C) CT angiography showing the anomalous artery arising from the thoracic aorta. (D) CT angiography images showing the venous drainage (arrow) to the pulmonary veins. (E–F) 3D CT reconstructions showing the anomalous artery.
This case combines an unusual association of congenital malformations – IL-PS and CCAM – with an infrequent diagnosis in adulthood. Few cases are described in literature of CCAM and IL-PS association.2,5–7 We found reports of presenting in adulthood were even scarcer.6
These lesions are usually diagnosed prenatally or in the first years of life. Both can have a wide spectrum of presentations ranging from asymptomatic to neonatal death depending on the size of the mass and consequent physiological impairment.1,2 After the first year of life recurrent infections are the most common presentation for both pathologies.1–4 It is important to note that potential for malignant transformation has been described in both entities, with stronger evidence in CCAM.3 Imaging has an important part in diagnosis of these lesions. Before birth ultrasound has a key role and after birth CT scan is the gold standard. Morphological features, location and blood supply can help distinguish the different malformations but this differentiation can be particularly difficult. Final diagnosis is established by histological analysis.1–3 CCAM is found in most cases to be an isolated finding, although type 2 has been described with other anomalies.1,2 Whereas the existence of further congenital anomalies, such as lung, cardiac, diaphragm or chest wall anomalies, is more frequent in PS, particularly in cases of EL-PS, it only happens in 15% of IL-PS cases.1,5 The mainstay of treatment for CCAM and PS is surgical resection, lobectomy is the usual procedure of choice.1–3 The authors present this case because it is so rare. Although uncommon and infrequently diagnosed in adults, congenital malformations should be included in the differential diagnosis of repeated infections, particularly if they are on the same lobe.
Conflicts of interestThe authors have no conflicts of interest to declare.