Approximately 5% of infants born with a meconium-stained amniotic fluid (MSAF) develop meconium aspiration syndrome (MAS).
AimThe aims of this study were to analyse demographic data, morbidity and mortality associated with MAS and to identify possible risk factors.
MethodsRetrospective chart review of newborns with MAS delivered at a tertiary center from January 1st, 1997 to December 31st, 2008.
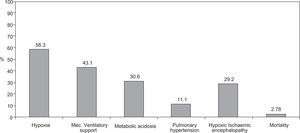
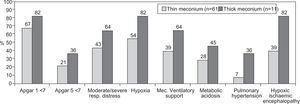
ResultsMAS was responsible for 1.4% of all Neonatal Intensive Care Unit (NICU) admissions, with a trend towards a decreasing incidence during the duration of the study, especially in the cases of thin meconium. Seventy two newborns were analysed during the study period: 55.6% (n = 40) were of the female gender, 62.5% were delivered by caesarean section, 93% had > 36 weeks of gestational age and 91.2% had a birth weight over 2500 g. Sixty-nine percent had an Apgar score < 7 at 1 minute and 23.6% an Apgar score < 7 at 5 minutes; foetal bradicardia was present in 26.4% of the newborns and tachycardia in 1.4%. The presence of meconium was associated with severe asphyxia and carried a bad prognosis with an increased risk of developing hypoxia (58.3%), need of mechanical ventilatory support (43.1%), respiratory and/or metabolic acidosis (30.6%), pulmonary hypertension (11.1%) and hypoxic ischemic encephalopathy (29.2%). The mortality rate was 2.8%. Thick meconium was associated with higher morbidity and mortality rates.
ConclusionsThe number of admissions for MAS has been decreasing mostly because of a lower admission rate due to thin meconium; the number of cases with thick meconium has remained constant throughout the years. An Apgar score < 7 at 1 minute and signs of foetal distress during labour were associated with MAS. The MAS related morbidity remains significant.
Aproximadamente 5% dos recém-nascidos com evidência de mecónio no líquido amniótico desenvolvem a síndrome de aspiração meconial (SAM).
ObjectivosConhecer os dados demográficos, a morbilidade e mortalidade na dependência da SAM e identifi car possíveis factores de risco.
MétodosEstudo retrospectivo dos recém-nascidos com SAM nascidos num hospital terciário entre 1 de Janeiro de 1997 e 31 de Dezembro de 2008.
ResultadosA SAM foi responsável por 1,4% das admissões na Unidade de Cuidados Intensivos Neonatais (UCIN), verificando-se uma tendência para o decréscimo no número de internamentos ao longo dos anos, principalmente dos casos com líquido amniótico tingido de mecónio. No período de estudo foram analisados 72 recém-nascidos: 55,6% do sexo feminino, 62,5% com parto por cesariana, 93% com idade gestacional > 36 semanas e 91,2% com peso ao nascimento > 2500g. 69% dos recém-nascidos apresentaram Índice de Apgar < 7 no 1.° minuto e 23,6% Índice de Apgar < 7 no 5.° minuto; bradicardia fetal foi observada em 26,4% dos recém-nascidos e taquicardia em 1,4%. A presença de mecónio no líquido amniótico condicionou o desenvolvimento de hipóxia (58,3%), necessidade de ventilação mecânica (43,1%), acidose respiratória e/ou metabólica (30,6%), hipertensão pulmonar (11,1%) e encefalopatia hipóxico-isquémica (29,2%). A taxa de mortalidade foi de 2,8%. A presença de mecónio espesso esteve associada a maiores taxas de morbilidade e mortalidade.
ConclusãoO número de internamentos por SAM tem vindo a diminuir principalmente devido ao decréscimo das admissões por líquido amniótico tingido de mecónio, enquanto o número de casos de mecónio espesso tem permanecido constante ao longo dos anos. O Índice de Apgar < 7 no 1.° minuto e a presença de sinais de sofrimento fetal durante o trabalho de parto apresentaram relação com a SAM. A morbilidade associada à SAM permanece significativa.
Eight to fifteen percent of newborns show evidence of a meconium-stained amniotic fluid. 1–3 Most of them can develop an effective respiratory adaptive response, while about 5 % show signs of respiratory distress at birth, ranging from a delay and difficulty in starting spontaneous effective breathing movements to signs of aspiration and persistent hypoxia.2 This complication is known as the meconium aspiration syndrome (MAS).
In the last decades the incidence of MAS has been decreasing, which has been attributed to improved obstetric practices, including the avoidance of post-term pregnancy and caesarean deliveries prior to evidence of foetal distress.4 However, MAS is still a major cause of morbidity and mortality in the neonatal period 5,6 (the mortality rate of newborns with MAS was close to 50 % in the 1970s, but currently ranges between 5 % and 37 %).7,8 In this context, it remains a concern to both obstetricians and neonatologists.9
Recent large-scale randomized studies have failed to show a decreased incidence of MAS related to procedures such as amnioinfusion and oropharyngeal or tracheal aspiration. Since 2005, the recommendations of the American Heart Association and the Neonatal Resuscitation Program only advocate intubation for tracheal aspiration of meconium at delivery for those infants with evidence of amniotic fluid with meconium and depressed vitality, respiratory distress, heart rate less than 100 beats per minute or decreased muscular tonus.10
The objectives of this study were to determine the incidence of MAS among the newborns delivered in a tertiary hospital, to identify risk factors associated with MAS, to analyze the therapeutic strategies used in these neonates (antibiotics, need for supplemental oxygen, use of surfactant, invasive versus non-invasive ventilation, use of high frequency ventilation and nitric oxide) and to determine the morbidity and mortality associated with MAS.
Material and methodsA retrospective clinical chart review of a newborn cohort admitted in a tertiary Neonatal Intensive Care Unit (NICU), from January 1st, 1997 to December 31st, 2008, with a MAS diagnosis was conducted.
Prior to 2005, delivery room guidelines in the presence of a meconium stained amniotic fluid mandated the aspiration of the newborn, often with recourse to tracheal intubation for aspiration, regardless of newborn vitality. Since then, the delivery room protocol has been based on the Neonatal Resuscitation Program of the American Academy of Pediatrics/American Heart Association guidelines, and doesn't advocate specific resuscitation manoeuvres to newborns presenting with a good vitality at birth; if the newborn is not vigorous (defined as the presence of respiratory effort, decreased muscle tone and decreased heart rate below 100 beats per minute) tracheal aspiration is recommended, prior to other resuscitation procedures.
Data regarding the gender, gestational age, type of delivery, birth weight, signs of foetal distress as tachycardia or bradycardia, Apgar score at first and fifth minute, consistency of meconium, and morbidity associated with MAS - including hypoxia, need for mechanical ventilatory support, pulmonary hypertension, respiratory and/or metabolic acidosis and hypoxic-ischemic encephalopathy - were collected and analyzed. The definitions of hypoxia (oxygen saturation below 94 %), respiratory acidosis (pH < 7.25 with PCO2 > 60mmHg), metabolic acidosis (pH < 7.25 with normal PCO2 and base deficit > 5), pulmonary hypertension (increased pulmonary vascular resistance and right-left shunt through the foramen ovale and/or patent arteriosus ductus, causing arterial hypoxemia even with FiO2 100 %) and hypoxic-ischemic encephalopathy (neurodevelopmental disorders following perinatal asphyxia) were previously established.
The severity of the respiratory distress observed in the newborns was classified based on the Silverman index, including mild (index 1–3), moderate (index 4–6) or severe respiratory distress (index 7–1).
All chest-radiographs were analysed and the treatment strategies used in these newborns were evaluated, including: antibiotics, oxygen supplementation, additional use of surfactant, use and type of ventilation (invasive versus non-invasive), use of high frequency ventilation and nitric oxide.
MAS was defined as the development of respiratory distress in the presence of meconium in the amniotic fluid, without other apparent cause.9 The diagnosis of hypoxic-ischemic encephalopathy was based on the modified Sarnat et Sarnat Score.11
The study parameters were evaluated through a descriptive statistic analysis; the chi-square test was utilized to analyze the trend in the incidence of MAS over the years.
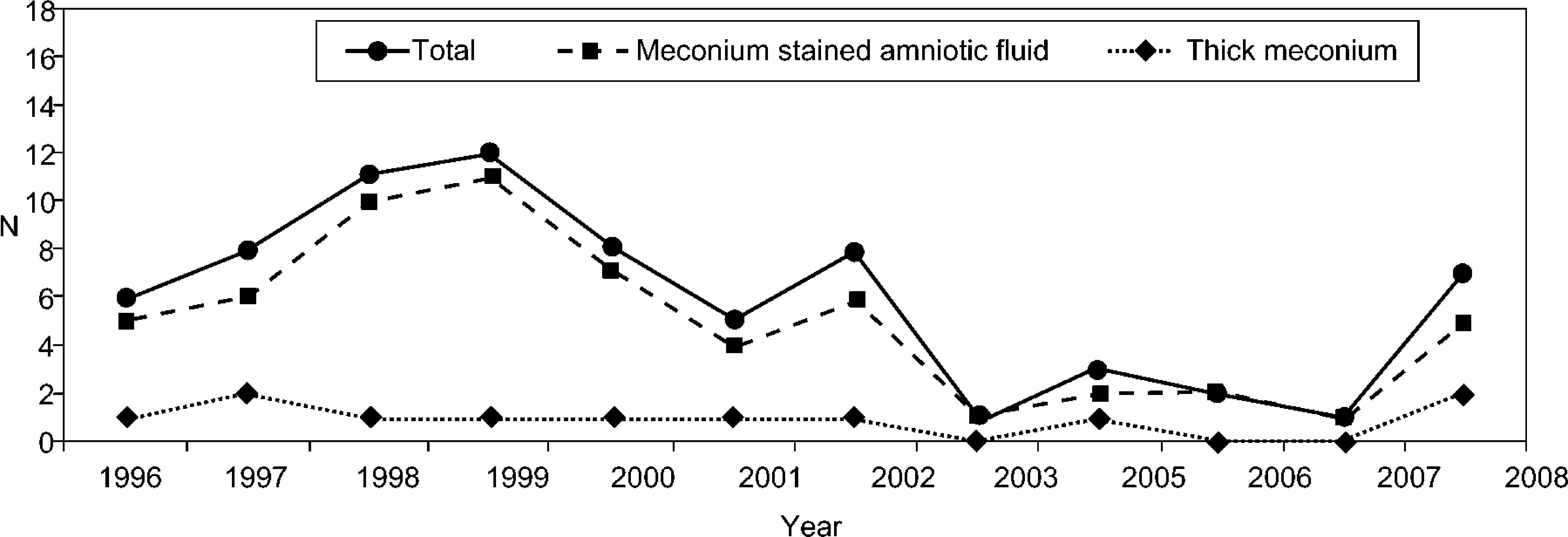
ResultsA total of 33 320 newborns were delivered during the study period, and MAS complicated 72 of these (0.22 %), accounting for 1.4 % of all NICU admissions. Except for the final year, there was a progressive decrease in the number of admissions for MAS throughout this 12-year period (Fig. 1) The chi-square test for the MAS incidence trend over the years showed a significant decrease (P = .03). The number of newborn admissions with evidence of meconium stained amniotic fluid decreased in parallel with the total number of admissions (Fig. 1), but the number of hospitalizations due to thick meconium remained the same.
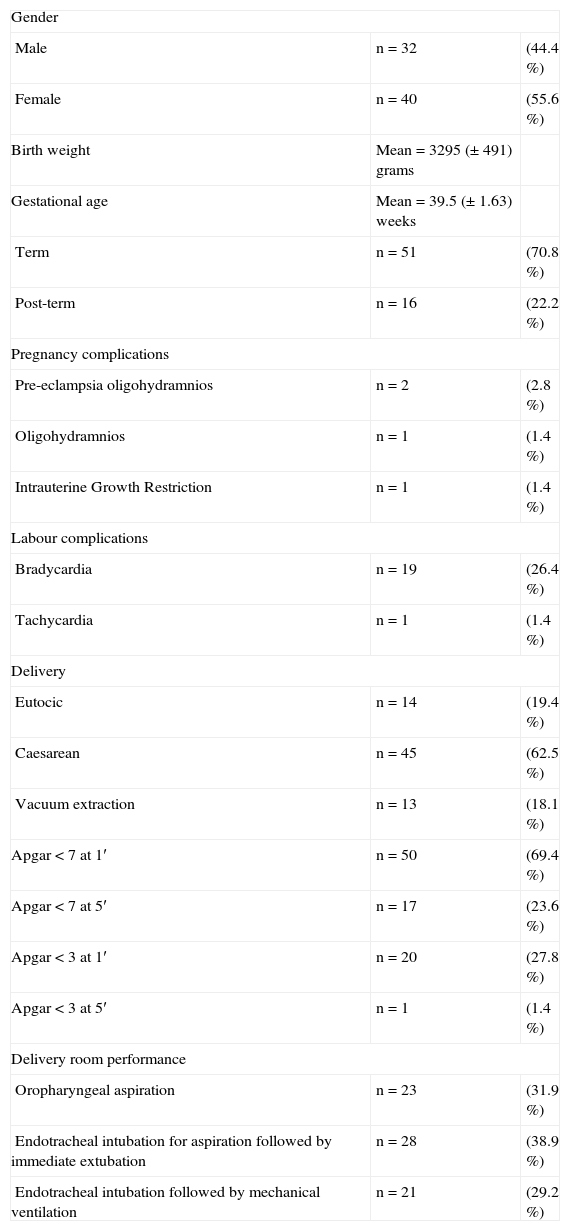
The demographic parameters of the studied population are shown in Table 1.
Study sample (n = 72) demographic data
| Gender | ||
| Male | n = 32 | (44.4 %) |
| Female | n = 40 | (55.6 %) |
| Birth weight | Mean = 3295 (±491) grams | |
| Gestational age | Mean = 39.5 (±1.63) weeks | |
| Term | n = 51 | (70.8 %) |
| Post-term | n = 16 | (22.2 %) |
| Pregnancy complications | ||
| Pre-eclampsia oligohydramnios | n = 2 | (2.8 %) |
| Oligohydramnios | n = 1 | (1.4 %) |
| Intrauterine Growth Restriction | n = 1 | (1.4 %) |
| Labour complications | ||
| Bradycardia | n = 19 | (26.4 %) |
| Tachycardia | n = 1 | (1.4 %) |
| Delivery | ||
| Eutocic | n = 14 | (19.4 %) |
| Caesarean | n = 45 | (62.5 %) |
| Vacuum extraction | n = 13 | (18.1 %) |
| Apgar < 7 at 1′ | n = 50 | (69.4 %) |
| Apgar < 7 at 5′ | n = 17 | (23.6 %) |
| Apgar < 3 at 1′ | n = 20 | (27.8 %) |
| Apgar < 3 at 5′ | n = 1 | (1.4 %) |
| Delivery room performance | ||
| Oropharyngeal aspiration | n = 23 | (31.9 %) |
| Endotracheal intubation for aspiration followed by immediate extubation | n = 28 | (38.9 %) |
| Endotracheal intubation followed by mechanical ventilation | n = 21 | (29.2 %) |
Meconium stained amniotic fluid was observed in 61 (84.7 %) cases and thick meconium in 11 (15.3 %).
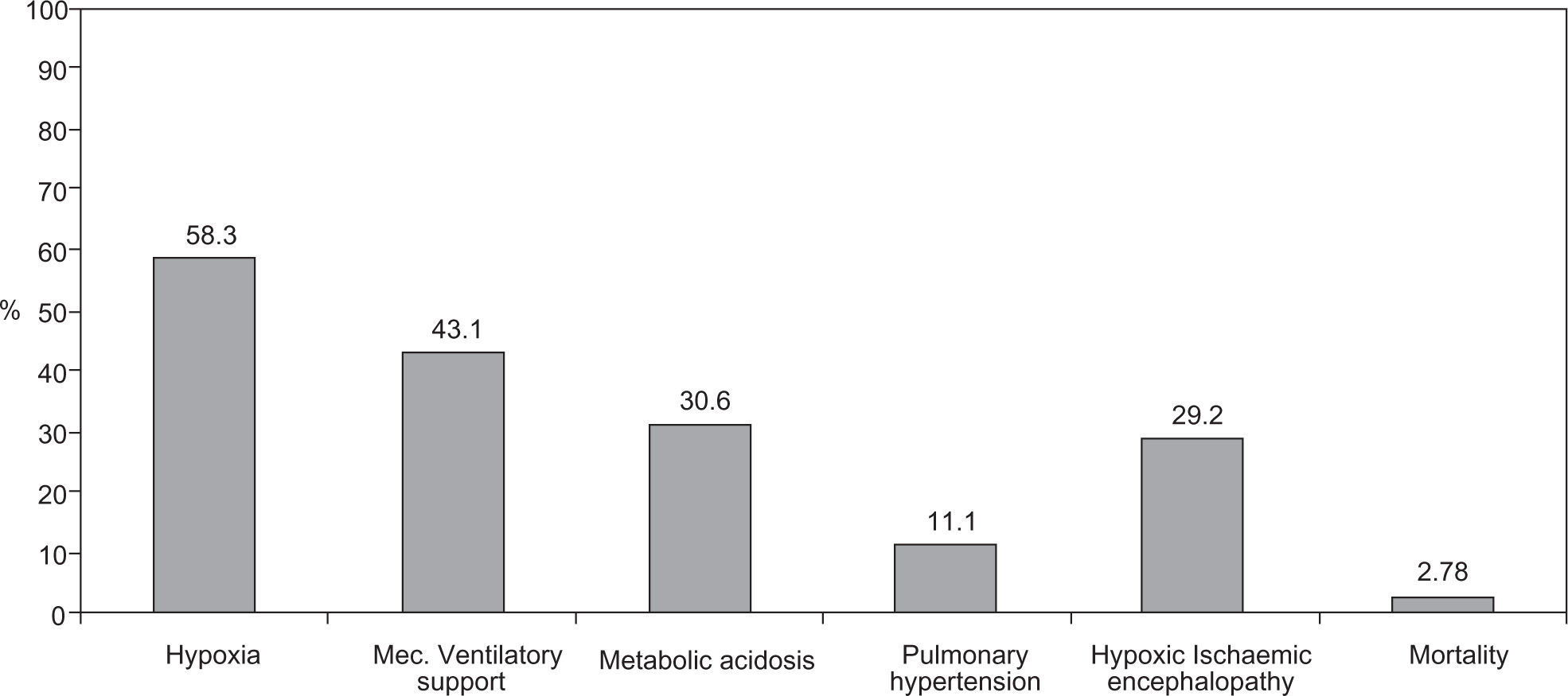
Of the 72 newborns identified with MAS, 11 (15.3 %) were born asymptomatic, developing respiratory distress during their first hours of life, while the remaining 61 (84.7 %) revealed signs of mild (38.9 %), moderate (23.6 %) and severe respiratory distress (22.2 %) at birth. MAS originated different types of morbidity including: hypoxia (58.3 %), need for ventilatory support (43.1 %), respiratory and/or metabolic acidosis (30.6 %), pulmonary hypertension (11.1 %) and hypoxic-ischemic encephalopathy (29.2 %) (Fig. 2).
Radiographic abnormalities were observed in 51 (63.9 %) newborns: interstitial infiltrates (n = 39), hyperinflation (n = 5), pneumothorax (n = 4), consolidation (n = 2) and pneumomediastinum (n = 1). Nineteen (26.4 %) newborns presented a normal chest radiography.
Antibiotic therapy was initiated in 53 (82.8 %) newborns. Of the newborns with MAS, 31 (43.1 %) required ventilatory support, 6 (8.3 %) only needed nasal Continuous Positive Airway Pressure (CPAP) and 25 (34.7 %) had to have invasive ventilation. Surfactant therapy was used in 6 (8.3 %) newborns and inhaled nitric oxide was necessary in one case.
The mean NICU's hospitalization period was 7.4 days with a median of 6 days (1–29 days). The mortality rate was 2.8 % (n = 2).
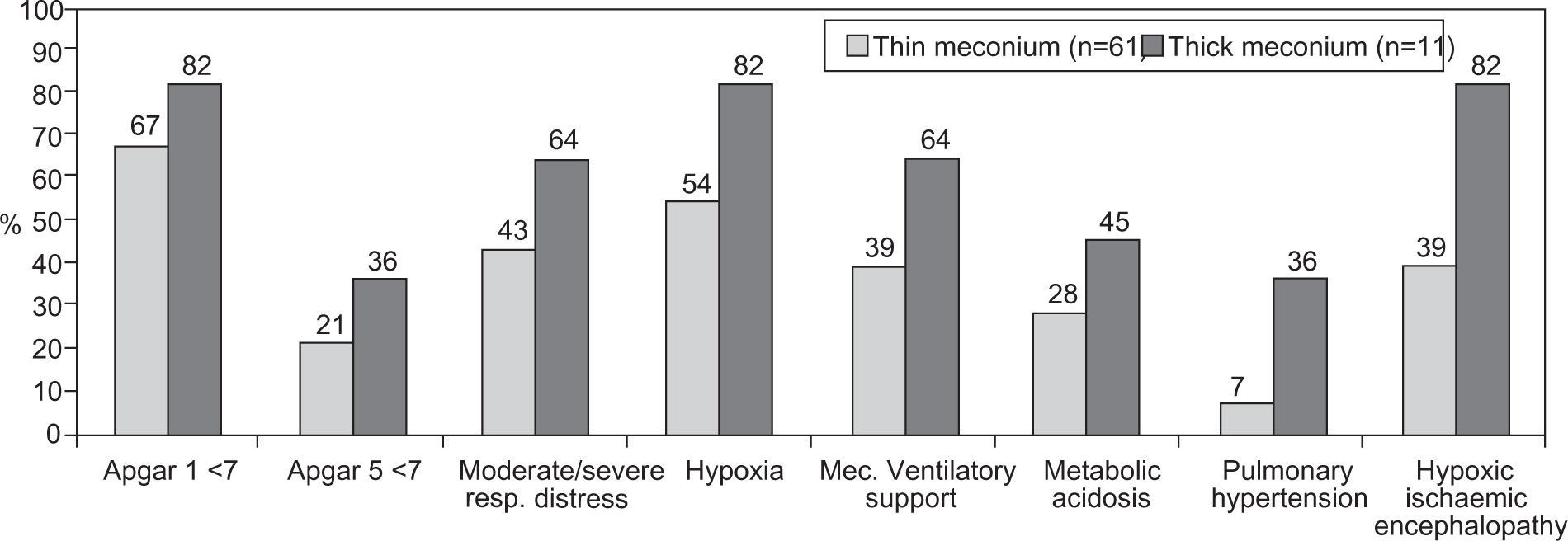
Comparing the group of patients with meconium stained amniotic fluid with the group with thick meconium, there was a greater morbidity in the second group (Fig. 3). One death was observed in each of the referred groups.
DiscussionMAS is based on airways blockage by the aspirated meconium, hindering ventilation and respiratory gases exchange, surfactant dysfunction with decreased lung compliance, respiratory tree mucosal inflammation with potential chemical pneumonia and pulmonary hypertension.4,7,10
In this population of 72 newborns (2.2/1,000 live births) MAS was a neonatal period complication and accounted for 1.4 % of NICU's admissions. Throughout this 12-year period a progressive decrease in the number of NICU's admissions for MAS was observed, in association with the progressive improvement of obstetric and peri-natal clinical care and the alteration of the delivery room protocol in the presence of meconium. Concomitantly with the decreasing number of MAS admissions, a lower number of hospitalizations associated with meconium stained amniotic fluid was observed; however, the prevalence of hospitalizations of newborns with evidence of thick meconium was the same. These data reflect the change in the delivery room practice, with performance of cardiopulmonary resuscitation only in the presence of newborns with impaired vitality at birth.
One of the limitations of this study, related to its retrospective nature, is the unknown number of births with evidence of meconium in the amniotic fluid but without MAS.
MAS has been described in association with foetal distress (suggested by foetal heart rate irregularities or a low Apgar score),12,13 gestational age greater than 40 weeks, caesarean section delivery and black populations.4 As described in various studies that attempted to address MAS risk factors, a high proportion of post-term pregnancies (22.2 %), signs of foetal distress during labour (27 %) and first minute Apgar score below 7 (70.3 %). was observed in this population. Some studies have suggested a four-fold decrease in the incidence of MAS with the decrease in post-term births.10 Foetal monitoring is essential, as well as a prompt obstetric intervention in the presence of evidence of foetal distress. In this context, foetal pulse oximetry is a new modality of antenatal foetal surveillance.
The imaging study confirms the diagnosis of MAS and usually reveals a diffuse interstitial infiltrate alternating with areas of hyperinflation.4,10 Newborns with severe disease can present X-ray images of consolidation or atelectasis, suggested as poor prognosis indicators, and about 15–33 % of cases develop complications such as pneumothorax or pulmonary emphysema.4,10 The studied population corroborates the imaging findings described in literature.
About one third of newborns with MAS require mechanical ventilation and sometimes high-frequency ventilation, nitric oxide, surfactant administration and extracorporeal membrane oxygenation (ECMO).3,4,13 In this population, MAS therapy was based mainly on antibiotics and ventilatory support when justified: 58.3 % of the newborns developed hypoxia and 85.0 % showed breathing difficulty signs; mechanical ventilation was necessary in 43.1 % of cases.
In the delivery room, intubation for meconium tracheal aspiration followed by immediate extubation was performed in 28 (38.9 %) newborns, while intubation followed by mechanical ventilation support was used in 21 (29.2 %). During NICU's hospitalization it was necessary to institute ventilatory support in 10 newborns that were initially in spontaneous ventilation. Of the total 31 (43.1 %) ventilated newborns, nasal CPAP was used in 6 (8.3 %) cases and conventional invasive ventilation in 25 (34.7 %). Conventional ventilation was considered when the supplemental oxygen concentration needed exceeded 70 %, and with evidence of apnea or respiratory acidosis. No case required the utilization of high-frequency ventilation. Surfactant was used in only two cases and nitric oxide in one. Several studies have described that the use of surfactant in the first six hours of life may improve oxygenation and thereby reduce pulmonary morbidity.10,14 Indeed, in newborns with MAS, endogenous surfactant is inactivated by meconium, resulting in atelectasic areas, decreased volume and lung compliance and impairment of oxygenation. Inhaled nitric oxide, a selective pulmonary vasodilator, was used in a newborn with an immediate presentation of respiratory distress, hypoxia and hypotonia, who developed severe pulmonary hypertension. MAS is an entity that may be associated with a significant morbidity and mortality, demanding treatment options that are not always available, such as ECMO.
Despite the therapy instituted, morbidity was significant and hypoxia (58.3 % of cases) was the most frequent complication, followed by the need for ventilatory support (during resuscitation) (43.1 % of newborns), metabolic acidosis (30.6 %), pulmonary hypertension (11.1 %) and hypoxic-ischemic encephalopathy (29.2 %).
Although the mortality rate observed (2.8 %) was lower than that reported in the literature, it may have been undervalued by the small sample analyzed. Studies reporting the experience of different neonatal centres have shown mortality rates ranging between 5 % and 37 %. 7,8 The two deaths observed occurred in 2001 and 2003, both in the first 48 hours of life. As described in the literature, deaths were related to respiratory complications and development of pulmonary hypertension: one newborn had a hypertensive pneumothorax, and the other presented diffuse pulmonary infiltrates associated with severe pulmonary hypertension.
ConclusionThe number of NICU's admissions for MAS has been declining due to a decrease in the number of newborn hospitalizations for meconium stained amniotic fluid, but the number of admissions by thick meconium has remained stable.
The presence of meconium in the amniotic fluid, foetal distress signs during labour and an Apgar score < 7 at first minute seem to be associated with MAS.
Despite the treatment options available in most neonatal care units, MAS continues to be responsible for a significant morbidity and mortality rate.
The perinatal monitoring of high risk newborns - those with evidence of meconium in the amniotic fluid - the timely choice for a caesarean delivery in the presence of signs of foetal distress, the decrease in the number of post-term pregnancies, the endotracheal intubation for meconium aspiration and the resuscitation manoeuvres provided for newborns with decreased vitality and cardio-respiratory depression are important measures for reducing the incidence of MAS and its associated morbidity and mortality.
Conflict of interestAuthors declare that they don't have any conflict of interest.