Despite all the benefits already published of GeneXpert® MTB/RIF (Xpert) for tuberculosis (TB) diagnosis, which is one of the top ten causes of death, according to the World Health Organization,1 there are a few studies comparing different pulmonary specimens - spontaneous sputum (SS), induced sputum (IS) and bronchoalveolar lavage (BAL). This study aims to analyse the Xpert accuracy in these samples for TB diagnosis and for rifampicin resistance identification in a tertiary TB reference centre in Brazil.
This retrospective study involved 1772 samples of patients who underwent bronchoscopy, SS collection or IS procedures. Each positive sample was simultaneously tested with Xpert, Acid-Fast Bacillus (AFB) smear using the Ziehl-Neelsen method and mycobacterial culture with drug susceptibility test (DST) processed by BACTEC Mycobacterial Growth in Tube® 960 or Lowenstein Jensen medium. Mycobacterial culture and DST were considered gold-standard methods.
The results were registered in a database at the Mycobacterial Laboratory of the Thorax Disease Institute, Federal University of Rio de Janeiro, a tertiary hospital in Brazil, between January 2017 and November 2019.
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy and likelihood ratios (LR) were calculated. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) 21.0® software.
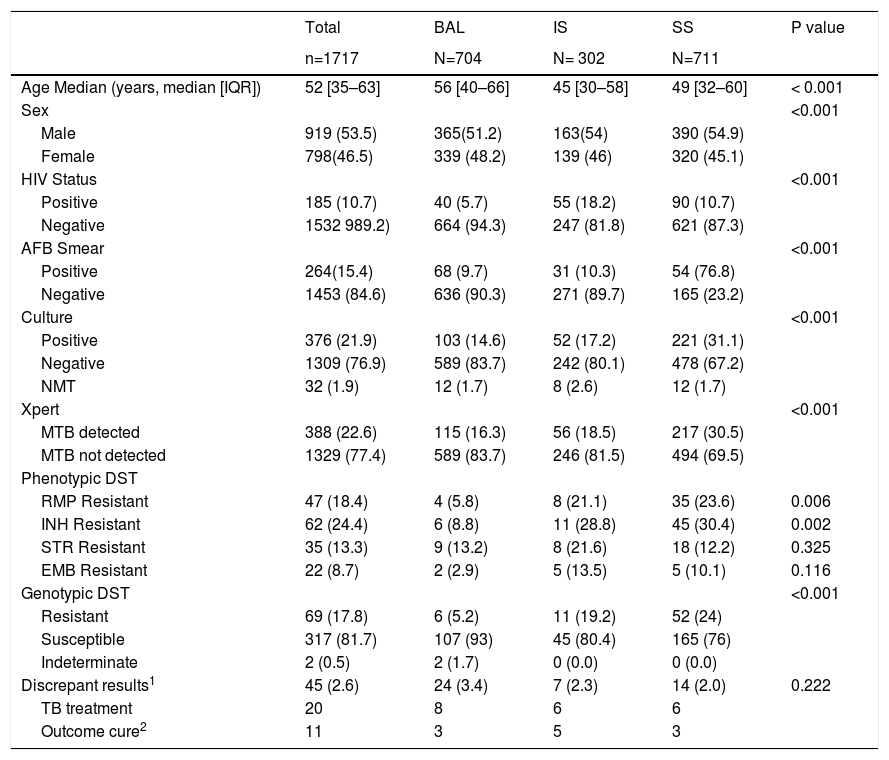
Of the 1772 pulmonary samples screened, 1717 (97%) were enrolled, and 55 were excluded from analysis (1 indeterminate Xpert and 54 contaminated cultures). Table 1 summarizes the demographics and clinical characteristics of the patients.
Demographic and clinical characteristics of patients.
| Total | BAL | IS | SS | P value | |
|---|---|---|---|---|---|
| n=1717 | N=704 | N= 302 | N=711 | ||
| Age Median (years, median [IQR]) | 52 [35–63] | 56 [40–66] | 45 [30–58] | 49 [32–60] | < 0.001 |
| Sex | <0.001 | ||||
| Male | 919 (53.5) | 365(51.2) | 163(54) | 390 (54.9) | |
| Female | 798(46.5) | 339 (48.2) | 139 (46) | 320 (45.1) | |
| HIV Status | <0.001 | ||||
| Positive | 185 (10.7) | 40 (5.7) | 55 (18.2) | 90 (10.7) | |
| Negative | 1532 989.2) | 664 (94.3) | 247 (81.8) | 621 (87.3) | |
| AFB Smear | <0.001 | ||||
| Positive | 264(15.4) | 68 (9.7) | 31 (10.3) | 54 (76.8) | |
| Negative | 1453 (84.6) | 636 (90.3) | 271 (89.7) | 165 (23.2) | |
| Culture | <0.001 | ||||
| Positive | 376 (21.9) | 103 (14.6) | 52 (17.2) | 221 (31.1) | |
| Negative | 1309 (76.9) | 589 (83.7) | 242 (80.1) | 478 (67.2) | |
| NMT | 32 (1.9) | 12 (1.7) | 8 (2.6) | 12 (1.7) | |
| Xpert | <0.001 | ||||
| MTB detected | 388 (22.6) | 115 (16.3) | 56 (18.5) | 217 (30.5) | |
| MTB not detected | 1329 (77.4) | 589 (83.7) | 246 (81.5) | 494 (69.5) | |
| Phenotypic DST | |||||
| RMP Resistant | 47 (18.4) | 4 (5.8) | 8 (21.1) | 35 (23.6) | 0.006 |
| INH Resistant | 62 (24.4) | 6 (8.8) | 11 (28.8) | 45 (30.4) | 0.002 |
| STR Resistant | 35 (13.3) | 9 (13.2) | 8 (21.6) | 18 (12.2) | 0.325 |
| EMB Resistant | 22 (8.7) | 2 (2.9) | 5 (13.5) | 5 (10.1) | 0.116 |
| Genotypic DST | <0.001 | ||||
| Resistant | 69 (17.8) | 6 (5.2) | 11 (19.2) | 52 (24) | |
| Susceptible | 317 (81.7) | 107 (93) | 45 (80.4) | 165 (76) | |
| Indeterminate | 2 (0.5) | 2 (1.7) | 0 (0.0) | 0 (0.0) | |
| Discrepant results1 | 45 (2.6) | 24 (3.4) | 7 (2.3) | 14 (2.0) | 0.222 |
| TB treatment | 20 | 8 | 6 | 6 | |
| Outcome cure2 | 11 | 3 | 5 | 3 |
Data presented as n of patients (%).
RIF= rifampicin; INH= isoniazid; STR= streptomycin; EMB= ethambutol.
Among the 704 BAL samples, 103 were culture positive, and 91 of them tested Xpert positive. Of the 601 culture negative samples, 24 were Xpert positive. These discrepant samples included 3 patients who had recently been treated for TB, suggesting nonviable fragments of MTB detected.
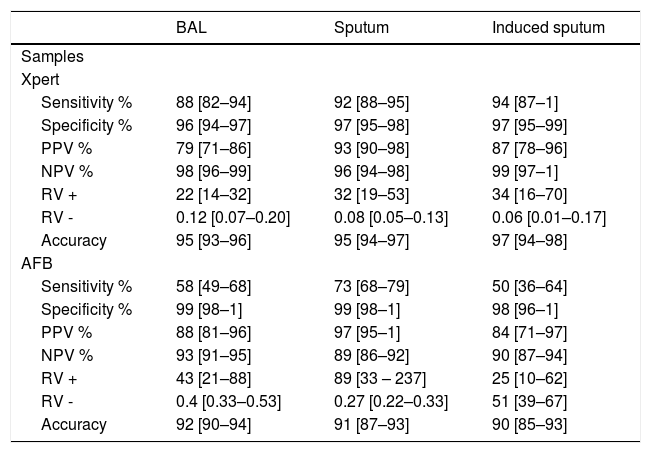
The sensitivity, specificity, NPV, PPV, accuracy and LR ratios for BAL are described in Table 2.
Performance value of Xpert MTB/RIF and AFB for respiratory samples compared to culture.
302/1717 of the specimens were of the IS (17.5%), and the Xpert performed on these samples registered a sensitivity of 92% and a specificity of 97%.
There were 10 (3.3%) discordant results when Xpert and cultures in IS were compared. Of these, 7 were Xpert positive and culture negative, among which 6 patients were treated for TB, and 5 completed treatment (Table 1).
Among HIV-infected patients (55/302 patients), sensitivity was 71%, and specificity was 97%. The performance of Xpert in IS is described in Table 2.
The sensitivity and specificity of Xpert in SS were 94% and 97%, respectively. Based on the higher prevalence of TB among patients submitted to SS collection, this sample had a higher PPV than BAL and IS (Table 2). There were 14 samples with negative culture and with Xpert positive results. Among these patients, 6 were treated for TB (Table 1).
The performance of Xpert was confirmed in several studies, but only a few compared it in three different specimens that can be used for pulmonary TB diagnosis. In our study, we included 1717 respiratory samples and confirmed its good performance in pulmonary samples, especially in IS.
These results are in line with Luo et al.,2 who showed that IS had a similar sensitivity, specificity and overall accuracy compared to BAL samples for TB diagnosis.
Notably, the high number of IS samples showed in our study has never been published before, with a sensitivity of 94% and a specificity of 97%. When compared with AFB, the sensitivity increased from 50% to 94%, and the likelihood of making an early diagnosis and prompt treatment increased. Chew et al.,3 conducted in Singapore in hospitalized patients (3) evaluated the diagnostic performance of pooling two IS specimens into one microbiological test with the addition of Xpert. The sensitivity and specificity of the molecular test in that study were 88.4% and 100%, respectively.
Fifty-five (18.2%) of the IS samples were from HIV-positive patients, with a sensitivity and specificity of 71% and 97%, respectively. Lower accuracy in seropositive sputum samples has already been shown in other studies.4
Xpert sensitivity on BAL was 88%, which was much higher than AFB sensitivity (58%) obtained in the same samples. Our results are consistent with a previous study, in which the sensitivity varied from 80% to 84.5%.5,6
The PPV of the BAL Xpert was moderate (79%), like Theron et al.7 showed, implying that false positives were common in patients who screened positive. In our study, this might be because some patients had various indications for undergoing bronchoscopy, and in Brazil, we have a high prevalence of TB.
The limitations in our study were mostly attributed to its retrospective design and to the lack of access to clinical and radiological information. We did not have access to all HIV serologies but only to positive HIV patients who were notified by the monitoring system for people with HIV. Furthermore, bronchoscopy was performed for various indications, not only for suspected TB, which may have reduced the PPV. At the time of our study, the new version of Xpert Ultra was not available, and as with other diagnostic tests, the high sensitivity was offset by a lower specificity.
In our study, the analysis of Xpert in IS showed a high accuracy, proving to be a useful test for TB diagnosis in patients with no spontaneous sputum and an alternative to bronchoscopy, because it is safe, inexpensive, less invasive and leads to minimal risks of complications than the latter.
FundingThis study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number: 306057/2020-4 to FCQM]. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Ethics approvalThe study was approved by the Institutional Ethics Committee under Protocol # 01561018.3.0000.5257.
Informed consentNot applicable.
The authors would like to thank the administration and staffs of the Mycobacterial Laboratory of the Thorax Disease Institute and patients of Clementino Fraga Filho Hospital.