The purpose of this series is to report the initial ECMO experience of the Neonatal Intensive Care Unit of Hospital de São João. The first three clinical cases are reported. Case report 1: a 39 weeks gestational age girl with severe lung hypoplasia secondary to a bilateral congenital diaphragmatic hernia. Case report 2: a 39 weeks gestational age girl with a right congenital diaphragmatic hernia and a tracheal stenosis. Case report 3: a 34 weeks gestational age boy, with 61 days of life, with a Bordetella pertussis pneumonia, severe pulmonary hypertension, shock, hyperleukocytosis and seizures.
O objetivo desta série é apresentar a experiência inicial da Unidade de Cuidados Intensivos Neonatais do Hospital de São João com ECMO no recém-nascido. São apresentados os 3 primeiros casos. Caso 1: recém-nascido de 39 semanas de idade gestacional, com hipoplasia pulmonar severa secundária a hérnia diafragmática congénita bilateral. Caso 2: recém-nascido de 39 semanas de idade gestacional, com hérnia diafragmática congénita direita e estenose traqueal. Caso 3: pré-termo de 34 semanas de idade gestacional, sexo masculino, com 61 dias de vida, com pneumonia por Bordetella pertussis, hipertensão pulmonar severa, choque, hiperleucocitose e convulsões.
Extra corporeal membrane oxygenation (ECMO) describes prolonged extracorporeal cardiopulmonary support for acute reversible respiratory and/or cardiac failure unresponsive to maximal conventional medical management.1
The first neonate successfully treated with venoarterial bypass was in 1975 by Bartlett’ group, and was treated for severe meconium aspiration syndrome.2
In Portugal, ECMO was used for the first time in the newborn in 2010, following the start of the adult ECMO program at Hospital de São João, in 2009. In 2012, two other newborns were treated with ECMO for respiratory failure.
The purpose of this series is to report the initial ECMO experience of the Neonatal Intensive Care Unit of Hospital de São João. The first three clinical cases are reported.
Case report 1A 39 weeks gestational age girl, birthweight 2520g, was born by C-section to a 41 years old, five gesta gravida. At 25 weeks of gestation, the diagnosis of left congenital diaphragmatic hernia (CDH) with liver up and severe lung hypoplasia was made on obstetrical ultrasound and by magnetic resonance image (MRI). Fetal echocardiographic exam was normal, karyotype was 46,XX, and no other structural anomalies were detected. The couple expressed to the obstetrical team their desire for full investment in the pregnancy and that ECMO should be offered after birth, if necessary.
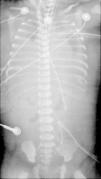
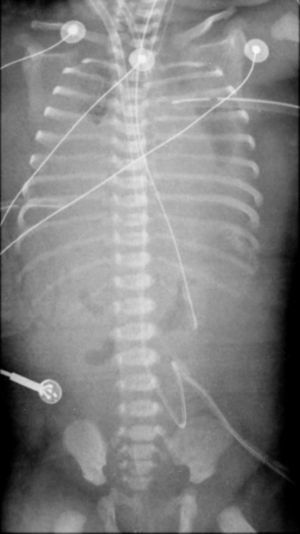
The baby was born in September 2010. She was immediately intubated after birth, the Apgar score (1st and 5th minutes) was 3/6, and she was started on mechanical ventilation (SIPPV; frequency=60min−1; inspiratory pressure=25cmH2O; FiO2=1.0). The initial arterial blood gas sample after admission to the neonatal intensive care unit (NICU) reported abnormal values: pH=6.8, PaO2=43mmHg, PaCO2=99mmHg, HCO3=16; BE=−26; lactate=6mmol/L. She was changed to high frequency oscillatory ventilation (HFOV) and started inhaled nitric oxide (20ppm). The echocardiogram 2D revealed a normally structured heart and severe pulmonary hypertension. The baby presented systemic arterial hypotension and inotropic and vasopressor support was started with dopamine+dobutamine. The oxygenation index was 44 and venoarterial ECMO (VA-ECMO) was started at hour five of life, with normalization of pH and blood gases. She underwent surgical correction of the left diaphragmatic defect with prosthesis, on day two of life. The chest X-ray never showed air filled lungs (Fig. 1). The baby was kept on VA-ECMO for 16 days, when the team decided to stop treatment, after several failed trials to wean from ECMO. The baby died and the autopsy revealed not a left but a bilateral diaphragmatic defect and confirmed very small volume with histological characteristics of hypoplastic lungs.
Case report 2A right CDH with liver up was diagnosed at 31 weeks of gestation in a female fetus of a 23 years old, two gesta gravida. The echocardiogram 2D was normal, and no other anomalies were detected on MRI. The gravida was referred to Hospital de São João and the ECMO team was contacted at parents’ request. A 39 weeks gestational age girl, birthweight 2900g, was born by vaginal delivery. The Apgar score was 4/6 (1st and 5th minutes), the baby was immediately intubated in the delivery room, and transported to the NICU under mechanical ventilation. The initial arterial blood revealed abnormal values: pH=7.21; PaCO2=64mmHg; PaO2=40mmHg; with significant ventilatory settings (frequency=60min−1; inspiratory pressure=25cmH2O; FiO2=1.0). She was started on HFOV, with transient improvement of respiratory acidosis. VA-ECMO was started at 20h of life when an oxygenation index of 47 was registered. The surgical repair was done with prosthesis on day four of life. After the start of active bleeding by the chest drain, a trial to wean from ECMO was successful in day seven of life.
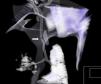
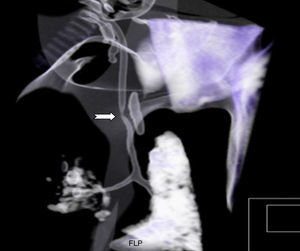
In day eight of life, the clinical state was characterized by a progressive worsening respiratory course with hypoxia, maintained after increasing ventilatory settings. She was changed to HFOV, and nitric oxide (20ppm) was started owing to severe pulmonary hypertension, hypoxia and an oxygenation index over 20. The chest X-ray revealed opacification of both lungs and the tracheal aspiration probe was difficult to advance along the endotracheal tube. A chest 3D computed tomography was performed to clarify the tracheal anatomy, which revealed a stenosis in its distal portion (Fig. 2). The baby was, by this time, extremely hypoxic, and after a multidisciplinary meeting, including neonatology, otorhinolaryngology, pediatric surgery, pediatric pneumology, thoracic surgery and the ECMO team, the decision was made not to offer corrective surgery of this second major congenital anomaly, which would have had to be performed under a second ECMO run, and also because of the severely hypoxic clinical state. The baby was placed on palliative care and died on day eight of life. The autopsy revealed a sling of the left pulmonary artery as the cause of a tracheal stenosis. The tracheal rings 2mm above the carina were complete and stenotic.
Case report 3On day 61 of life (two weeks corrected age) a 34 weeks gestational age boy was transferred to Hospital de São João NICU, because of Bordetella pertussis pneumonia with severe pulmonary hypertension, shock and seizures. His twin sister had died on day 43 of life of B. pertussis and Parainfluenza 3 co-infection with an overwhelming fulminant course. Our patient, had been in close contact with his twin sister, and postexposure antimicrobial prophylaxis with azithromicin was offered. Nevertheless, the baby presented with cough and coryzal symptoms and was admitted to another NICU, where the polymerase chain reaction (PCR) test of respiratory secretions was positive for B. pertussis and Parainfluenza 3. The clinical picture was a deteriorating one, and invasive mechanical ventilation was needed because of respiratory failure. The cardiological exam did not reveal pulmonary hypertension at that time. The laboratorial study revealed hyperleukocytosis of 50.5×109L−1, and a double volume exchange transfusion was performed, with transient effect. A progressive deteriorating clinical picture developed, marked by seizures, systemic arterial hypotension, pneumonia and severe pulmonary hypertension. The baby was treated with phenobarbital, dopamine+dobutamine, maximal ventilatory settings, and FiO2 before being transferred to our NICU.
At admission, the cardiological exam revealed severe pulmonary hypertension with an oxygenation index of 25. Inhaled nitric oxide (20ppm) and sildenafil were started, with immediate, but transient response. Over the following days there was a progressive deteriorating respiratory and hemodynamic course and VA-ECMO was started on day five after admission to our NICU because of lack of response to maximal conventional medical management. After an eight day ECMO run, the baby was weaned and mechanically ventilated for a further 18 days. He was treated with a 14 days course of erythromycin and because of suspected bacterial superinfection a course of vancomycin and meropenem was associated until biochemical markers of infection became negative. The a-EEG brain monitoring revealed multiple seizures, treated with phenobarbital, levetiracetam and lidocaine. The cranial ultrasound revealed severe periventricular hyperecogenicity, edema and enlargement of lateral ventricles. The MRI performed before discharge revealed multiple encephaloclastic lesions and of the white subcortical matter and ischemic lesions of the basal ganglia. Forty-four days after admission, the baby was transferred back to the original hospital.
DiscussionIn our series, two patients died and one survived. According to the Extracorporeal Life Support Organization (ELSO) registry, neonates continue to have the best survival across all patient diagnoses. Since Bartlett’ first report of survival of the sickest neonates after ECMO support, several clinical studies have shown its efficacy.3–8
ECMO for the treatment of CDH was first reported in 1977, by German et al.9 Today, many centers worldwide use ECMO for the treatment of CDH, with variable results.10 A standardized postnatal management of infants with CDH, the CDH EURO Consortium Consensus, has been proposed in 2010.11 In this Consensus, criteria for ECMO eligibility are well defined.
Bilateral diaphragmatic hernias are uncommon (2%–5%) and are usually fatal.12 ECMO was offered to the patient in case report 1 on the assumption that the prenatal diagnosis of left CDH was correct. Otherwise ECMO would probably not have been offered. Although the final outcome was the likely one, the insistence of the parents on keeping on ECMO after surgery, until they could be sure that the child was not viable, was taken into account.
Right-sided hernias seem to behave differently from the left sided hernias. Right-sided CDH infants have an increased benefit from ECMO but better survival rate entails a higher rate of chronic lung disease.13 The patient described in our case report 2 had a right-sided CDH, and although there was a good outcome from the ECMO run, the tracheal stenosis determined the final outcome. Pulmonary artery sling is frequently associated with tracheal and/or bronchial stenosis. A number of patients receive only re-implantation or relocation of the left pulmonary artery while other patients may require tracheoplasty for stenosis of the airway.14
B. pertussis infection may develop a fulminant course in very young, unimmunized infants, and is characterized by pneumonia that rapidly evolves to respiratory failure with refractory hypoxemia, extreme leukocytosis and cardiogenic shock requiring cardiovascular support.15–21 The setting up of refractory hypoxemia characteristically is rapid and it does not respond properly to advanced ventilation maneuvers, including high-frequency oscillatory ventilation, inhaled nitric oxide, and ECMO.22,23 There are some hypotheses for this poor outcome. An association between high WBC count and increase in severity led to the theory that vascular infiltration or hyperviscosity may be a factor in inducing PH and heart failure.22,16
Encephalopathy associated with pertussis infection is rare, occurring in 0.5–1% of all cases, and the diagnosis is suggested by seizures with pertussis infection, in the absence of other diagnosis. It may be the result of the direct neurologic actions of toxins, effects of hypoxia, hemorrhages, vascular occlusions and latent virus infection. However, the pathomechanisms of central nervous system complications are not fully understood.24,25
Survival and prognosis of children supported with ECMO depends on the initial pathology, criteria for starting ECMO and clinical status, as well as associated congenital anomalies. A correct evaluation of fetuses with CDH, with an assessment of the degree of pulmonary hypoplasia and associated congenital anomalies, is mandatory before starting ECMO.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors are grateful to all medical and nursing staffing from the Neonatal Intensive Care Unit of Hospital de S. João, for all the commitment in the care of our ECMO patients. The authors are also in debt to the ECMO specialist team from the Hospital S. João ECMO Center, for the dedication in the development of our ECMO program.