COVID-19 related Acute Respiratory Failure, may be successfully treated with Conventional Oxygen therapy, High Flow Nasal Cannula, Continuous Positive Airway Pressure or Bi-level Positive-Pressure ventilation. Despite the accumulated data in favor of the use of different Non-invasive Respiratory therapies in COVID-19 related Acute Respiratory Failure, it is not fully understood when start, escalate and de-escalate the best respiratory supportive option for the different timing of the disease. Based on the current published experience with Non-invasive Respiratory therapies in COVID-19 related Acute Respiratory Failure, we propose an algorithm in deciding when to start, when to stop and when to wean different NIRT. This strategy may help clinicians in better choosing NIRT during this second COVID-19 wave and beyond.
Non-invasive respiratory therapies (NIRT) have become paramount interventions in the management of COVID-19 induced acute respiratory failure.1 Patients developing COVID-19 related Acute Respiratory Failure may be successfully treated by means of Conventional Oxygen Therapy (COT), High Flow Nasal Cannula (HFNC), Continuous Positive Airway Pressure (CPAP) or Bi-level Positive-Pressure ventilation (Bilevel-PAP) with the avoidance of endotracheal intubation (ETI) and invasive mechanical ventilation (IMV).
Recent data from the world´s widest database2 (International Severe Acute Respiratory and emerging Infections Consortium-ISARIC) suggests that 20% of patients with COVID-19 are admitted at some point of their illness into an intensive care unit (ICU) or high dependency unit (HDU). Non-invasive ventilation (including both CPAP and Bi-level PAP) was applied in 15% of cases, while High-flow nasal cannula (HFNC) in 14%.
Two main concerns dealing with the use of NRST are the risk of delaying intubation in case of failure and the fear of virus spreading among health care workers (HCW) during noninvasive respiratory treatment.
The pragmatic implications for the clinical practice are respectively the applications of reliable models predicting NIRT failure3 and the use of proper personal protective equipment (PPE) together with equipment and ventilator setting limiting viral particles dispersion.4,5 Contactless monitoring can help minimize entry of HCW into rooms and so decrease their risk of viral load exposure.6
It is well known from pre-COVID 19 data, that the recipe for the success of NIRT include mandatorily the management of the carefully selected candidate in an expert setting by means of a well trained staff, with the choice of a proper equipment and bearing in mind that there is a tight window of opportunity warranting short-interval monitoring and considering timely intubation for worsening respiratory status. Ideally, NIRT, like interventional pulmonology procedures, should be performed in a dedicated negative pressure room with strict isolation precautions and sufficient ventilation to avoid aerosol contamination.7
Prolonging NIRT in absence of clinical-arterial blood gases improvement may expose the patient to the risk of an unduly delayed intubation with an increased mortality.
Since the SARS epidemic, some reservations have been raised about the benefits of NIRT, the risks of aerosolization and the consequent increase in infections among HCW. Recently, several publications report that NIRT are not actually aerosol generators, but aerosol dispersers.8 And so, with the appropriate PPE,4,5 and in the hands of experienced teams, it’s possible to reduce the intubation rate without seriously increasing the risk of nosocomial infections.
Recent experience in COVID-19 related Acute respiratory failure (21 publications including 1553 patients)9–27 with HFNC, CPAP and Bi-Level Ventilation (using negative pressure rooms in only one series15) shows average success of 60%, 55% and 59%, respectively, and an average infection rate in health professionals of 5.2%, less than the 12% reported in New York City among the staff not necessarily working with NIRT.28
However there is significant heterogeneity in the employed NIRT protocols followed in the published studies.
Recent data,29 in invasively mechanically ventilated patients, demonstrated the existence of different phenotypes of COVID-19 induced ARDS depending on static compliance, ventilator ratio as surrogate of dead space, and D-Dimer levels (as surrogate of diffuse thrombo-embolic pulmonary disease). The phenotype showing higher D-Dimer levels and lower static lung compliance is associated with greater mortality rate. One could speculate that the likelihood of improvement in gas exchange (I.e. PaO2/FiO2 ratio) after NIRT is greater in the earlier phases of COVID-19 induced ARDS patients who are still on spontaneous breathing when lung mechanics is preserved and pulmonary vascular thrombo-embolic diseases is less like to have developed. However, further clinical large controlled studies are needed to demonstrate this issue.
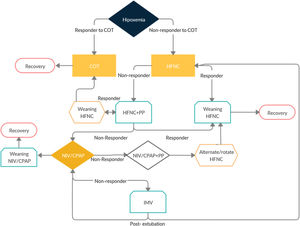
Based on this current evidence we would like to propose an algorithm (Fig. 1 and Table 1) and specific criteria to start NIRT, when to escalate NIRT, when to wean NIRT and when to proceed to Intubation. We believe it could help clinicians uniformize their practice of the management of Hypoxemic Respiratory Failure of COVID-19 and help to homogeneously collect data for the forthcoming studies.
Summary of the proposed algorithm.
| Step 1-Start COT when SpO2< 92% | ||||
| Venturi mask to target SpO2 92-96% | ||||
| Step 2-Start HFNC when PaO2/FiO2 <300 on O2>5 L/min | Step 3-Wean HFNC | Step 2-HFNC Failure | Step 9-HFNC After extubation | Step 10-Wean HFNC After extubation |
| Ramp up from 30 L/min until 60 L/min of Flow; FiO2 to maintain SpO2>93% | Decrease FiO2 first; when you reach FiO2 40% decrease flow | If ROX is below 2.85 at 2h, below 3.47 at 4h; or below 3.85 at 12h | If PaCO2< 45 during SBT or intubation not associated with COPD | If Flow 30 L/min and FiO2 30% |
| Step 4-Start CPAP when PaO2/FiO2 <200 | Step 5-Wean CPAP | -CPAP Failure | Step 8-NIV failure | |
| Apply 10 cmH20 and FiO2 to maintain SpO2 > 93% | When SpO2 > 94% with FiO2 < 50% and CPAP ≤ 5cmH20 | If PaO2/FiO2 <100 or 20% increase in PaCO2 | If HACOR Index > 5 1h or 12h after starting therapy | |
| Step 6-Start NIV when PaO2/FiO2 <100 and RR≥30 and/or respiratory distress under CPAP Or PaCO2> 45mmHg | Step 8-NIV failure | Step 9-NIV after extubation | ||
| If HACOR Index > 5 1h or 12h after starting therapy | If PaCO2> 45 during SBT or intubation associated with COPD | |||
| Consider Self-Proning after Step 1,2,4 and 6 as tolerated by the patient, and if efficacious extend it during 3−5 days. | ||||
Surprisingly, no published studies took into consideration normalized PaO2 and normalized PaO2/FiO2 ratio in hypoxemic and hypocapnic patients.30 As a great deal of COVID-19-related pneumonia patients show hypoxemic hypocapnic alkalosis, SpO2 values may underestimate the severity of lung damage because of the left-side shift of hemoglobin dissociation curve. We speculate that PaO2/FiO2 ratio may not be considered a reliable score of hypoxemia if we do not consider PaO2 values standardised to PaCO2 levels in hypocapnic patients (1,66*PaCO2- PaO2 – 66,4 mmHg). Again in this scenario the severity of lung impairment may be underestimated by stratifying patients according to conventional PaO2/FiO2 ratio with the consequent risk of delaying the time for starting or escalating a NIRT or for intubation.
Criteria to start conventional oxygen therapy (COT)- a)
Start when SpO2 < 92% (Room air)
- b)
Use Venturi mask to target spO2 of 92−96%31 (choose preferentially masks with filter media covering the exhalation ports like the Intersurgical FiltaMask ™-Fig. 2)
- c)
In case the patient needs a higher oxygen concentration, use a non-rebreather reservoir mask
Reevaluate after 4 h: If SpO2 and Respiratory rate-RR (<30) remain stable-Maintain COT (Responder)
If SpO2 drops ≥ 2% or RR > 30, proceed to Arterial Blood Gas (ABG) analysis (NON-Responder) >> escalate to HFNC
Criteria to start HFNCIt is not clear if HFNC can be more beneficial if started earlier. Preliminary non-peer review experience seems to point into an earlier start.32
- a)
Early start: If PaO2:FiO2 < 300 or SpO2 < 93% on O2 > 5 L/min or SpO2 < 94% with FiO2 40%33
- b)
Late start: SpO2 < 92% under O2 at 15 L/min and or PaFiO2 < 15023
- c)
Start with: Ramp up from 30 L/min until 60 L/min to accustom the patient (40–50 if not tolerated); FiO2 to maintain spO2 > 93%; Temperature 31−37º according to patient’s comfort
- d)
A surgical mask should be placed over the nose and mouth of a patient with a properly fitted nasal cannula before initiating therapy. It not only reduces droplet deposition34 but also improves COVID-19 patient’s oxygenation35 without any clinically relevant side effect, except for a moderate reduction in CO2 clearance.34 This may require increasing flow rate of HFNC if the patient is displaying increased work of breathing.
Follow ROX Index (SpO2/FiO2: RR) criteria36,37: In brief calculate ROX at 2 h, 6 and 12 h. If its value is increasing then the patient´s respiratory status is improving.
If at 2 h: ROX is between 2,85−4.87 increase support and re-evaluate in 30 min aiming at an improvement in ≥0.5; if ROX is ≥4.88 continue treatment; if ROX is below 2.85 consider ETI or escalate to short trial of CPAP or NIV before (see steps 4−6).
If at 6 h: ROX is between 3.47–4.87 increase support and re-evaluate in 30 min aiming at an improvement in ≥0.5; if ROX is ≥4.88 continue; if ROX is below 3.47 consider ETI or escalate to short trial of CPAP or NIV before (see steps 4−6).
If at 12 h: ROX is between 3.85–4.87 increase support and re-evaluate in 30 min aiming at an improvement in ≥0.5; if ROX is ≥4.88 continue; if ROX is below 3.85 consider ETI or escalate to short trial of CPAP or NIV before (see steps 4−6).
Three recent studies applying the ROX index in COVID-19 patients, suggest different cut-off points like slightly higher values ≥4,94 at 2 and 6 h38 and ≥5,37 at 4 h39 and lower values ≥3,7 at 6 h.23 Moreover other authors point to a daily significant decrease in the ROX index as a predictor failure within the first 3 days of HFNC treatment.25
Criteria of HFNC weaningDecrease FiO2 hourly aiming at SpO2 92–98%. When you reach FiO2 of 40%, decrease flow until weaning.40 However an ideal weaning protocol remains to be established. A current RCT41 is studying 3 different protocols: 1) gradually wean flow by 10 L/min/h. When it reaches 20 L/min, FiO2 reduction will then begin at 0.1 /h until it reaches 0.3. 2) gradually reduce FiO2 by 0.1 /h until it reaches 0.3. At this point, flow will be reduced by 10 L/min/h until it reaches 20 L/min 3) both flow and FiO2 will be gradually reduced simultaneously at a rate of 10 L/min and 0.1 /h, respectively, until they reach the HFNC weaning off targets (20 L/min for flow and 0.3 for FiO2).
Criteria to start CPAP- a)
If PaO2:FiO2 < 200 or PaO2 < 60 mmHg or RR > 30 (while on oxygen or HFNC)
- b)
If PaO2:FiO2 < 300 or SpO2 < 93% on O2 > 5 L/min- and Patient has BMI > 30 (OPTIONAL)16
If you start CPAP analyze improvement in PaO2:FiO2 in 1 h: a) if improves ≥15%14 or ≥30%20 consider existence of LUNG RECRUITABILITY
Criteria of CPAP weaning (a weaning trial should be attempted every day to avoid a delay in CPAP removal)Patients on helmet CPAP who do not show signs of respiratory distress (e.g. RR < 25) and maintain a SpO2 > 94% with a FiO2 < 50% and a PEEP< = 5 cmH2O undergo a weaning trial. Patients maintaining a PaO2:FiO2 ratio >250 on Venturi mask with a FiO2 < 40% for at least 24 h are considered successfully weaned from helmet CPAP.20
OR
Reduce Helmet CPAP level to the minimum possible (5–6 cmH20) maintaining a FiO2 not higher than 50%. If de-recruitment is absent and the PaO2/FiO2 ratio is stable as compared with higher PEEP levels the patients is ready to undergo a CPAP weaning trial.42
Criteria to start NIV- a
PaO2:FiO2 < 100 and RR ≥ 30 and or respiratory distress under CPAP
- b
Suggested parameters PEEP 12–16 cmH20 and PS set with the aim of VT 4−6 ml/kg and FiO2 aim of target SpO2 90–95%.43
- c
In patients with hypercapnic respiratory failure (PaCO2 > 45 mmHg)
- a
Between NIV sessions, to allow for feeding and rest, HFNC could be delivered during 1 h with a flow of 50 L/min and a FiO2 to achieve adequate oxygenation (SpO2 ≥ 92%).
INTUBATION criteria after CPAP and NIVIndication for intubation includes the presence of either ≥1 major or ≥2 minor criteria lasting for ≥1 h20:
Major criteria: respiratory arrest, respiratory pause with unconsciousness, severe hemodynamic instability (i.e., SBP < 90 mmHg instead of adequate volume resuscitation), and intolerance to helmet CPAP leading to discontinuation of the device.
Minor criteria: reduction of ≥30% of basal PaO2:FiO2 ratio, PaO2:FiO2 ratio <100, 20% increase of PaCO2 if basal PaCO2 was ≥40 mmHg, worsening of alertness, new onset or persistent respiratory distress, SpO2 < 90%, and exhaustion.
OR
According with Brusasco et al.,26 If after 4 h of CPAP, PaO2/FiO2 decreasing, RR ≥ 30, PaO2 < 60 mmHg.
OR
A deterioration in oxygen saturation with a PaO2/FiO2 < 150 or 175 mm Hg after 1 h of NIV, a respiratory rate > 30/min, a high APACHE score, and a HACOR score >5.44
OR
Also HACOR Index (Heart rate, Acidosis (pH), Consciousness (GCS), Oxygenation, and Respiratory rate (HACOR)-Table 2) >5, 1 h or 12 h after Initiating CPAP/NIV.45
HACOR Index.
| Variables | Category (j) | Assigned points |
|---|---|---|
| Heart rate, beats/min | ≤120 | 0 |
| ≥121 | 1 | |
| pH | ≥7.35 | 0 |
| 7.30−7.34 | 2 | |
| 7.25−7.29 | 3 | |
| <7.25 | 4 | |
| GCS | 15 | 0 |
| 13−14 | 2 | |
| 11−12 | 5 | |
| ≤10 | 10 | |
| PaO2/FiO2 | ≥201 | 0 |
| 176−200 | 2 | |
| 151−175 | 3 | |
| 126−150 | 4 | |
| 101−125 | 5 | |
| ≤100 | 6 | |
| Respiratory rate, breaths/min | ≤30 | 0 |
| 31−35 | 1 | |
| 36−40 | 2 | |
| 41−45 | 3 | |
| ≥46 | 4 |
OR
Also if ROX is below 2.85 at 2 h, below 3.47 at 4 h; or below 3.85 at 12 h
NIV/CPAP/HFNC after extubationIf suspected hypercapnia (PaCO2 > 45 mmHg during SBT) or intubated with associated COPD (or other cause of Chronic Hypercapnic Respiratory Failure) extubate to NIV.46 Also in case of failure of first Spontaneous Breathing Trial -SBT (with PEEP 6cmh20 FiO2 35% for 30 min, patients VT < 6 mL/kg of Predicted Body Weight, RR > 22 and SpO2 decreasing).47
According with the protocol of Thille AW et al.,48 NIV will be immediately initiated after planned extubation by prolonged sessions during the 48 h following extubation: a first session of at least 4 h, and then sessions of at least 2 h, during all the night (continuous NIV from 10 P.M. to 6 A.M.) if it is possible, for a total duration of at least 12 h a day. NIV will be performed with a ventilator dedicated for NIV (ICU ventilator with NIV mode or NIV ventilator) in pressure-support ventilation using the following ventilator settings: Minimal pressure-support level of 5 cm H2O targeting a tidal volume around 6–8 ml/kg, a PEEP level between 5 and 10 cmH2O, a FiO2 adjusted to obtain adequate oxygenation (SpO2 ≥ 92%), a pressure ramp slope between 0.1 and 0.2 s and a cycling off criterion at 25–30% of peak inspiratory flow.
Rotation of NIV/HFNC48: To allow better tolerance, between NIV sessions, HFNC will be delivered with a flow of 50 L/min and a FiO2 to achieve adequate oxygenation (SpO2 ≥ 92%). The treatment including HFNC and NIV will be delivered for at least 48 h after planned extubation. NIV in this setting will offer better ventilator assistance, while HFNC better tolerance and humidification.
If there is no hypercapnia during SBT nor the intubation was associated with COPD (or other cause of Chronic Hypercapnic Respiratory Failure), extubate to HFNC.46 If non-responsive to HFNC (see criteria of failure), rescue use of CPAP.
A recent case series report of a successful use of HFNC in extubating 4 COVID-19 patients and de-escalating from CPAP in 5.49
Moreover according with a recent meta-analysis,50 HFNC reduces work of breathing to a similar extent as NIV, while keeping similar PaCO2 values in hypercapnic patients. So a strategy using HFNC as integrated management for patients with COPD exacerbation could be proposed, as a valid alternative to COT during breaks of NIV or to facilitate NIV discontinuation and prevent discomfort and NIV failure.50
Extubation failure criteria51: RR < 10 or >30/min; Rapid Shallow breathing index (RR: VT in Liters > 100); SpO2 < 90% with FiO2 ≥ 40%, PaO2:FiO2 < 200, HR 120, hemodynamic instability, GCS < 8, agitation, coma, no cough reflex, ph < 7,30, Respiratory Distress.
Criteria for HFNC discontinuation post-extubationIn the absence of ARF symptoms 48 h after planned extubation, treatment can be stopped and switched to standard oxygen therapy, after a weaning test using HFNC with a flow of 30 L/min and FiO2 of 30%. In case of occurrence or persistence of ARF symptoms at 48 h, HFNC will be continued or reinitiated by periods of 24 h until disappearance of symptoms.
Prone-Positioning as a complementary tool to improve oxygenationCombination of early NIRT and prone position has been recently proposed as a measure to “buy time” and potentially improve outcomes.52
Self-proning can be tried with COT,53–55 HFNC,56–58 mask CPAP59 or Helmet CPAP60,61 to increase oxygenation.
These studies applied different frequency and duration of proning sessions, with one randomized prospective study aiming at 16 h/day having a non significant reduction in Intubation rate.56 However the majority of studies show a significant improvement in oxygenation and respiratory rate, with no major adverse events. There is some suggestion that patients with a PaO2/FiO2 ≤ 150 have the best improvement and that for those with PaO2/FiO2 > 200 4 h/day may be enough while those with a PaO2/FiO2 < 200, 10 h/day may be needed.62 Without a uniformized protocol it makes sense to apply self-proning as much as tolerated by the patient, and if efficacious extend it during 3−5 days.
Some authors suggest to encourage self-proning 2 times/day (at least 30 min until patient distress, monitoring SpO2 each 15 min) during the first 3 days.63 Others propose 1−2 hours 3–4 times a day during more than 5 consecutive days,64 and others to alternate every 2 h between prone and supine during the day and sleep in the prone position at night.65
According with Coppo et al., the best results come from early self-proning and patients with increase in inflammatory markers (LDH, CRP, Platelets).61
To increase tolerance some authors propose analgo-sedation with Alprazolam with or without hydroxizine66 or even Dexmedetomidine.67
NIRT management of COVID-19 related pneumonia, like other severe Pneumonia, should be performed only in highly protected settings such as ICU, RICU or HDU where a close and continuous monitoring of cardiopulmonary parameters, a careful assessment of clinical and physiologic early response to NIRT, and the availability for a quick escalation to intubation and invasive ventilation as well as for the management of respiratory and non respiratory complications are feasible and easily applicable. Un-monitored ward-based COVID-19 areas are not safe and inadequate for managing such critically ill and complex patients. Pre-COVID-19 expertise of the team on NIRT should be the strongest driver to choose (when it’s possible depending on the epidemiologic wave) the environment where allocate COVID-19 patients in the earlier stages of the diseases when the chance of success of NIRT in avoiding intubation is greatest.68 Considering the still limited availability of ICU beds and the strategic need to keep as free as possible this precious environment reserved for the admission only of more severly ill patients to be supported by means of invasive ventilation and extra-pulmonary lung dysfunctions (ie. renal, cardiovascular replacement) and complications (i.e. septic shock), the majority of the patients should be managed in COVID-19 RICU or ICU COVID-19 area by Pulmonologists and/or other expert physicians in NIRT.69 This is supported by the recent Italian experience, where the insufficient newtwork of RICU in pre-COVID-19 time may have caused the quick saturation of ICU beds during the tremendous outbreak in Lombardy. The following expanded network of pulmonologist units together with more restrictive national measures againts the virus dissemination has contributed to the mitigation of COVID-19 impact on mortality in the remaining Italian regions at the end of the first wave.70
In conclusion, protocolized use of NIRT through the proposed algorithm may help clinicians working in Respiratory Intermediate Care units to make choices and potentially decreasing admissions to the overwhelmed Intensive Care Units.
Conflicts of interestThe authors have no conflicts of interest to declare.