Non-tuberculous mycobacterial (NTM) infections are increasingly rapidly worldwide. The reason for this phenomenon is unclear, but may include the ageing population, the increasing use of immunosuppressive drugs, the increasing prevalence of diseases that confer susceptibility to NTM, such as COPD and bronchiectasis, and growing testing for NTM. Awareness of the NTM related diseases is rising but is still suboptimal. Guidelines from the American Thoracic Society and Infectious Diseases Society of America have provided a framework for evaluating disease and evaluating care. Compliance with these guidelines is, however, very poor globally.
NTM infections are amongst the most challenging cases that respiratory and infectious diseases physicians face. The challenges include intrinsic antibiotic resistance, complex drug regimens, poor tolerability and significant side effects associated with therapy and poor response rates. The decision to initiate treatment is therefore often difficult and requires careful evaluation of benefits and risks. Optimal management of NTM infections requires multidisciplinary care with close collaboration between physicians, microbiologists, physiotherapist/allied health professionals, primary care physicians and the patient.
There remains a need for greater research into the epidemiology, clinical evaluation and treatment of NTM pulmonary disease. Randomised clinical trials are now being conducted which may provide useful data on the effectiveness of some new and existing therapies.
In this review, we discuss the growing importance of NTM pulmonary disease and the opportunities for progress in clinical research for these conditions.
Nontuberculous mycobacterial pulmonary disease (NTM-PD), also referred to variously as NTM-pulmonary disease or NTM-lung disease is caused by infection with a range of pathogenic species of NTM.1–3 These diseases are relatively rare but place a major burden on patients and their physicians because they are complex to manage, requiring prolonged antibiotic regimens with a great deal of complexity in the microbiology, radiology, drug treatments, drug–drug interactions and other aspects of treatment.1,2 With these factors combined, NTM patients are among the most complex and challenging cases that pulmonologists face in clinical practice. NTM are also responsible for non-pulmonary disease and disseminated disease. These forms of the disease will not be discussed here.
The incidence and prevalence of these infections is rising alongside increases in the prevalence of predisposing diseases like chronic obstructive pulmonary disease (COPD) and bronchiectasis, and the increasing life expectancy of patients with cystic fibrosis.4–9 All pulmonary physicians need to know to suspect NTM pulmonary disease, how to recognise and diagnose it and to understand the priniciples of treatment.
In this concise review, we discuss the clinical aspects of NTM pulmonary disease with a focus on diagnosis and therapy.
EpidemiologyMore than a hundred NTM species have been described and the number is growing (www.bacterio.cict.fr/m/mycobacterium.html). Fortunately most of them have no clinical significance and relatively few are responsible for lung disease. NTM are ubiquitous in the environment and are readily identified in community and household water sources amongst other habitats.1 As a result, humans are constantly being exposed to NTM, but NTM-PD remains uncommon because only a small proportion of individuals appear to be susceptible.10–13 It is remarkable that in the past decade, data have been published from Europe, North America, Australia and Asia all reporting a consistent increase in the incidence and prevalence of NTM-PD.14–20 The extent to which this increase is real, or reflects increased testing and reporting is unclear. Unlike TB, NTM infection is not a reportable disease in the majority of countries which limits the quality and reliability of data collection.
Regional differences in the prevalence of NTM disease have been demonstrated around the world including 9.8 per 100,000 from Canada in 2010,14 8.6 per 100,000 from Oregon, USA15 or less than 1 in 100,000 from Brazil.16 NTM isolation appears to be highly prevalent in Asia by contrast with one study suggesting a national prevalence in Japan of between 33 and 65 per 100,000, predominantly due to Mycobacterium avium complex (MAC).17 Although estimates closer to those reported in Canada and US were found in a study from Taiwan.18
In Europe, a recent study from the UK identified a rising incidence of NTM isolation driven almost exclusively by an increase in MAC disease with incidence rising from 5.6 per 100,000 to 7.6 per 100,000 over the period 2007–2012.21 Ringshausen et al. reported a prevalence of 2.3 per 100,000 for NTM pulmonary disease in 2009 in Germany rising to 3.3 per 100,000 in 2014.22
Thus internationally the rates of NTM disease are therefore relatively similar, allowing for differences in the methods of reporting, the definition of cases and the different methods employed in the studies.
A large observational study in Europe identified important genographic variation in the species isolated from patietns with MAC being most frequent in Northern Europe (accounting for 44% of all NTM isolates vs 31% in Southern Europe) and a predominance of Mycobacterium xenopi in Southern Europe (accounting for 21% of NTM isolates in Southern Europe vs 6% in Nothern Europe).23
Thus NTM-PD remains a rare disease, but one of increasing importance. It is a problem worldwide and although the distribution of different species varies according to geographical location, MAC is the predominant species in nearly all studies.14–23
Clinical characteristicsNTM-PD is typically chronic, slowly progressive and difficult to diagnose. It can present at any age, but is most common in patients older than 50.24–27 NTM-PD is strongly associated with conditions that compromise pulmonary or systemic immunity such as bronchiectasis, COPD, cystic fibrosis or other chronic respiratory disorders and systemic immunodeficiency.28–34 The disease is often subclassified into nodular-bronchiectatic and fibrocavitary “phenotypes”. Although there is a high degree of overlap between these subtypes, they are clinically useful.24–27 In Europe and the USA the nodular-bronchiectatic phenotype patients are typically elderly causasian females while fibrocavitary disease is strongly associated with co-morbid COPD and is therefore more common in men.27
A distinct “morphotype” of individuals with nodular-bronchiectatic NTM-PD has been reported in the United States consisting of post-menopausal females with tall stature, low body mass index, scoliosis, pectus excavatum, mitral valve prolapse and middle lobe and lingula bronchiectasis. Post menopausal females often with middle and lingula nodular bronchiectactic NTM disease are referred to as “Lady Windermere syndrome”.35,36 A MAC-associated hypersensitivity pneumonitis-like illness has also been reported to occur following exposure to aerosolized MAC mainly in indoor hot tubs and is referred to as hot tub lung.7
The symptoms of NTM-PD are non-specific. Diagnosis is often delayed because symptoms may appear to be part of the underlying disorder, or may be misdiagnosed. Patients most frequently have cough, sputum productions, breathlessness, profound fatigue, weight loss and fever.
DiagnosisAs previously noted, NTM are present in the environment and particularly in environmental water sources. They may therefore contaminate sputum specimens or be transiently present in the airways without causing disease leading to “false positive” sputum samples. Therefore Isolation of an NTM species in clinical samples is frequently not associated with clinically significant disease.37 Critieria for diagnosis first proposed in the 2007 American Thoracic Society guidelines remain the standard used internationally.27 These require clinical, microbiological and radiological evidence of disease before the diagnosis can be confirmed. Specifically, two isolates of NTM from a non-sterile site (typically sputum) or a single bronchoalveolar lavage or lung biopsy specimen, symptoms attributable to NTM-PD and radiological evidence of NTM-PD are considered sufficient evidence for a diagnosis of NTM-PD (Table 1).
IDSA/ATS criteria for diagnosis of NTM- pulmonary disease.1,27
| Clinical features |
| Pulmonary symptoms attributable to NTM disease. |
| Radiology |
| Nodular or cavitary opacities on chest radiograph, or a high-resolution CT scan that shows multifocal bronchiectasis with multiple small nodules. |
| Appropriate exclusion of other diagnoses. |
| Microbiology |
| Positive culture results from at least two separate expectorated sputum samples; if the results are non-diagnostic, consider repeat sputum AFB smears and cultures. |
| or |
| Positive culture results from at least one bronchial wash or lavage. |
| or |
| Transbronchial or other lung biopsy with mycobacterial histopathological features (granulomatous inflammation or AFB) and positive culture for NTM or biopsy showing mycobacterial histopathological features (granulomatous inflammation or AFB) and one or more sputum or bronchial washings that are culture-positive for NTM. |
It is recommended that 2–3 early morning sputum samples on different days are used for diagnosis of NTM-PD.1,27 In patients with a high index of suspicion where spontaneous sputum has not been obtained it is appropriate to use sputum induction or bronchoscopy. The least invasive procedure that can achieve a definitive diagnosis is preferred.1 Once the sample is obtained it is stained for the presence of acid-fast bacilli, most frequently with Ziehl–Neelsen stain. This does not discriminate between Mycobacterium tuberculosis and NTM. The broad availability now of nucleic acid amplification (NAA) tests for MTB such as the Xpert MTB/RIF assay, however, allows a better distinction of species in the early phase of disease: a negative NAA gives a strong early indicator that a sample positive for AFB on stain is likely to contain an NTM.1,27,38,39 Culture remains the gold standard for diagnosis and is used for subsequent confirmation of the presence of NTM, species identification and drug susceptibility testing.
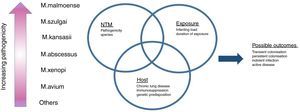
Mechanisms of diseaseNTM is an area of significant activity in terms of understanding basic mechanisms of disease. The outcome of pulmonary NTM disease is likely to reflect a complex interplay between the exposure (e.g. the infecting dose of NTM, the number of organisms and duration of exposure), the organism (virulence and pathogenicity) and the host (immune status, genetic risk factors, underlying chronic lung disease) (Fig. 1).40–44 To date, there is limited information to understand this complex interaction.
Some organisms are more pathogenic than others and are therefore more likely to cause clinically relevant disease.
Among the host risk factors, the impact of chronic lung disease is discussed below. Immunosuppression is a key risk factor, and during the early days of the HIV epidemic most literature regarding NTM concerned disseminated MAC infection as a manifestation of the acquired immunodeficiency syndrome.45 This is now exceptionally rare in the era of highly active anti-retroviral therapy. NTM infection is now more frequently encountered in the context of iatrogenic immunosuppression with drugs directed against tumour necrosis factor alpha or its receptor, corticosteroids or other systemic immunsuppressive drugs.46–48 Recent data suggests that inhaled corticosteroids (ICS) in COPD patients (and perhaps to a lesser extent asthma patients) increase the risk of NTM.49–51 A Danish case control study found ICS increased the risk of NTM by 29× among COPD patients, with high dose ICS increasing the risk to nearly 50×.49 Brode and colleagues recently extended these findings in a large population based study among 417,494 patients with obstructive lung disease. They found ICS increased the risk of NTM by 86%, with fluticasone containing drugs increasing the risk by more than double.51 This increases the evidence that ICS is associated with infectious complications in patients with COPD.52–55 The IL-12/interferon gamma pathway is thought to be critical to host defence against mycobacteria and inherited defects in this pathway are associated with NTM-PD and disseminated NTM.56 The extent to which apparently sporadic NTM is associated with defects in this pathway is not fully known.
Some of the first data from whole exome sequencing studies among NTM patients have been reported, including a novel susceptibility gene on chromosome 6 identified as the TTK protein kinase gene involved in mitosis and DNA repair.57–59 Another exome sequencing study implicated cystic fibrosis transmembrane conductance regulator (CFTR) and cilia related genes in the pathogenesis of NTM.59 Matsuyama et al. recently described the transcriptional response of the primary respiratory epithelial cell cultures to MAC and Mycobacterium abscessus infection. They found downregulation of genes involved in ciliary function, supporting a vicious cycle concept of NTM infection, and found M. abscessus tended to be more pro-inflammatory than MAC.60 Microbiological research is also ongoing. NTM have classically been considered an intracellular pathogen but Qvist showed in CF than M. abscessus can grow in biofilms.61 Ongoing research in this area is likely to add significantly to our understanding of the pathogenesis of NTM infections.
Although the exact route of NTM acquisition has not been well established, considering the broad distribution of NTM in the environment it is very likely that the organisms are ingested, inhaled or implanted. Aerosolization of small droplets is the probably cause of pulmonary disease. Unlike TB, person to person transmission has not been convincingly identified for the majority of NTM. Person to person transmission of M. abscessus has been identified among patients with cystic fibrosis.41,62
NTM and underlying diseasesNTM can be associated with virually any structural lung disease but is most frequently described in the context of COPD and bronchiectasis.63–66 Pneumoconioses such as silicosis are also strongly linked to NTM infection, although the prevalence of such diseases is rapidly falling worldiwde.63,64 A meta-analysis of observational studies suggests that 9% of bronchiectasis patients are infected with NTM.67 This summary statistics hides enourmous heterogeneity and includes rates varying from 0% to 60%.66–71 Henkle et al. reported on patient characteristics and treatment of bronchiectasis in the US bronchiectasis registry. Among 1247 patients with NTM, 83% were female, 19% had co-existing COPD, 22% were infected with Pseudomonas aeruginosa. ICS therapy was used by 36%.71 Patients are frequently infected with other organisms such as P. aeruginosa and there is also a link between NTM infection and Aspergillus disease, including invasive Aspergillosis.72–75 Among the large number of aetiologies associated with bronchiectasis, cystic fibrosis is the most commonly associated with NTM infection.76 NTM infections are increasing among CF populations worldwide. The reasons for this are unclear but this may reflect the increasing lifespan of patients and the greater success that has been achieved in controlling traditional bacterial infections like P. aeruginosa, as well as greater awareness leading to increased NTM diagnosis.77–79 Current estimates are that NTM infects between 4% and 32% of CF patients.76
There is also increasing interest in the frequent isolation of NTM in patients with previous or current M. tuberculosis infection. Other respiratory diseases commonly linked to NTM include pulmonary alveolar proteinosis, where autoantibodies against GM-CSF may impair host macrophage responses to NTM leading to infection particularly with MAC.80–83
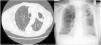
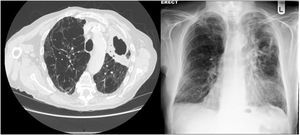
RadiologyAs noted for the clinical presentation, the radiological appearances of NTM-PD are varied and non-specific. There are two major radiological patterns of disease, notably fibrocavitary and nodular bronchiectatic disease, although it is noted that featues of both can overlap.24 The fibrocavitary form of the disease overlaps radiologically, and sometimes clinically, with the appearances of pulmonary TB. This disease is therefore often associated with cavities and areas of opacity located in the upper lobes, which may be associated with volume loss, pleural thickening and bronchiectasis. The cavities are most frequently described as “thin-walled” and other features of pulmonary TB are likely to be absent.27
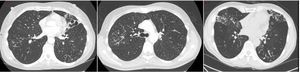
In nodular-bronchiectatic disease (Fig. 2) there is bronchial dilatation most frequently affecting the middle lobe and lingula, often associated with nodules and tree-in-bud opacities.1,27 There may be other features such as consolidation, and the severity of the radiological bronchiectasis may vary from cylindrical to cystic. Since NTM is thought to be both a cause and a complication of bronchiectasis it should be noted that any radiological appearance of bronchiectasis may be potentially associated with NTM. An example of fibrocavitary disease is shown in Fig. 3.
Nodular-bronchiectatic phenotypes of NTM. The CT images (left to right) show severe bronchiectasis in the Lingula in a patient with M. avium lung disease. Tree-in-bud is most easily visible in the right lung and the middle image shows this more clearly in the same patient. The right image shows a bilateral nodular bronchiectatic disease in a gentleman with M. abscessus infected.
In every patient, the decision to treat NTM-PD is potentially challenging and involves balancing the benefits of treatment and the risks of drug toxicity.27,84 The ideal scenario is that patients with NTM-PD are managed in experienced centres by physicians and multidisciplinary teams who manage large numbers of NTM patients and have access to the microbiological facilities and antibiotic susceptibility data required to make the most informed decisions.84 This likely to be the case for only a tiny minority of patients worldwide.
NTM-PD is usually a slowly progressive disorder and so the urgency to initiate treatment is not the same as in the case of pulmonary TB, unless the patients is immunocompromised or has invasive disease. Patients may be observed without treatment to determine whether disease is progressive radiologically or clinically.1,27
The goal of treatment is to improve the patients symptoms and their long term quality of life. This is an important statement, as there objective of NTM lung disease treatment is often described in textbooks and review articles as being to render the sputum negative by culture for NTM for 12 months which is important and the definition of microbiological response. Nevertheless, the initial discussion regarding whether to initiate treatment should take into account what the patient wishes to achieve with therapy, and not only a discussion of microbiological considerations.85–87
It is also essential to manage the patients underlying diseases such as COPD and bronchiectasis and careful control of other airway infections, including P. aeruginosa infection.88–90 Attention to airway clearance to control sputum and related symptoms, and prevention of respiratory infections through vaccination are as important as treatment of the NTM.88–90 In patients with bronchiectasis, attention should be paid to identifying any underlying cause and to treating this where appropriate.91 In patients with immunosuppression such as HIV or use of immunosuppressive drugs, the immunodeficiency should be reversed as far as possible.
Therapy is continued for at least 12 months after culture converts to negative (culture conversion).1,27 Prolonged regimens are required because relapse is common. A recent pilot study showed that typical NTM regimens lack early bacteriocidal activity in vivo, i.e. they fail to achieve reductions of bacterial load over the initial months of treatment. Prolonged therapy is therefore required to achieve cure.92
MAC therapyThe treatment of MAC lung disease is typically with combination therapy incorporating a macrolide (clarithromycin or azithromycin).1,27 The standard regimen includes a rifamycin (rifampicin or rifabutin), ethambutol and a macrolide. Patients will receive therapy for 18–24 months until sputum is negative by culture for 12 months or more. Patients without cavitary disease may expect the get a similar rate of microbiological cure with less cost and toxicity by taking three times per week therapy as demonstrated in recent observational cohort studies.93 No difference has been found between clarithomycin and azithromycin containing regimes.94
Fibrocavitary/cavitary disease is treated with daily therapy with recent guidelines also advocating the use of amikacin or streptomycin for 2–3 months in severe disease.1,27
The treatment of macrolide resistant or treatment refractory MAC lung disease is particularly challenging. Alternative therapies used include moxifloxacin, aminoglycosides, inhaled amikacin and clofazimine. Seeking expert advice in the management of these patients is strongly advocated.
Mycobacterium kansasiiCompared to MAC or M. abscessus complex (MABC), M. kansasii may be regarded as a readily treatable pathogen. It is usually sensitive to standard anti-TB drugs expect pyrazinamide. The recommend drug regimen is rifampicin 600mg, isoniazid 300mg and ethambutol 15mg/kg. Treatment is continued for 12 months after sputum conversion.
M. abscessus complexThis is more challenging and the therapy is typically individualised based on the results of drug susceptibility tests, potential drug–drug interactions and tolerability.1,27 Current guideline recommendations are shown in Table 2. Macrolide based therapy is recommended but data suggests it is more effective against Mycobacterium massiliense compared to M. abscessus due to the effect of inducible macrolide resistance.95 The overall success rate of therapy is low and therefore surgical resection should be carefully considered when the disease is localised sufficiently for this to be an option.
Antibiotic treatment suggestions. Definition of severe disease includes AFB smear positivity, cavitation, severe symptoms or evidence of systemic illness. Clarithromycin 1g daily is administered in at least two divided doses, e.g. 500mg twice daily. All therapies are continued until sputum is culture negative for at least 12 months.1,27
| Organism/severity | Antibiotic regimen |
|---|---|
| Non-severe MAC-PD | Rifampicin 600mg+ethambutol 25mg/kg+azithromycin 500mg or clarithromycin 1g per day, three times per week. |
| Severe MAC-PD | Rifampicin 600mg+ethambutol 15mg/kg+azithromycin 250mg or clarithromycin 1g daily. Consider intravenous or nebulised amikacin. |
| Clarithromycin resistant MAC-PD | Rifampicin 600mg daily, ethambutol 15mg/kg and isoniazid 300mg (+pyridoxine 10mg daily) or moxifloxacin 400mg daily. Consider IV or nebulised amikacin. |
| M. kansasii (rifampicin sensitive) | Rifampicin 600mg+ethambutol 15mg/kg+isoniazid 300mg (+pyridoxine 10mg daily) or azithromycin 250mg daily or clarithromycin 1g daily. |
| M. malmoense | Rifampicin 600mg+ethambutol 15mg/kg+azithromycin 250mg or clarithromycin 1g daily. Consider amikacin IV or nebulised in the case of severe disease. |
| M. xenopi | Rifampicin 600mg+ethambutol 15mg/kg+azithromycin 250mg or clarithromycin 1g+moxifloxacin 400mg daily or isoniazid 300mg (+pyridoxine 10mg) daily. Consider amikacin IV or nebulised in severe disease |
| M. abscessus (macrolide sensitive or inducible macrolide resistance) | Initial phase: At least 4 week initial phase of intravenous amikacin, tigecycline, imipenem and oral clarithromycin or oral azithromycin. Continuation phase: nebulised amikacin+oral macrolide in combination with 1–3 drugs selected based on drug susceptibility and tolerance (clofazimine, linezolid, minocycline/doxycycline, moxifloxacin/ciprofloxacin and co-trimoxazole. |
| M. abscessus (macrolide resistant) | Initial phase: At least 4 week initial phase of intravenous amikacin, tigecycline and imipenem. Continuation phase: nebulised amikacin in combination with 2–4 drugs selected based on drug susceptibility and tolerance (clofazimine, linezolid, minocycline/doxycycline, moxifloxacin/ciprofloxacin and co-trimoxazole. |
The taxomony of MABC has evolved in recent years with the identification of subspecies by whole genome sequencing MABC is now subdivied into at least 3 subspecies – M. abscessus subspecies abscessus, M. abscessus subsp. massiliense and M. abscessus subsp. bolletii.95–100 Alongside the revision of the classification of M. abscessus, the identification of inducible macrolide resistance has been an important development.97–100M. abscessus contains an erm gene encoding for a methyltransferase that methylates the site of action of macrolides within the 23s rRNA. As a result, the organism may appear suspcetible to macrolides early (i.e. at day 3) but is resistant after prolonged incubation (day 14).97–100 There is variation in erm function between subspecies of the abscessus genus, specifically M. bolletii and abscessus typically possess erm gene sequences associated with inducible macrolide resistance while the erm gene is dysfuncton in M. massiliense leading to macrolide susceptibility.97–100
Important issues with NTM therapyCompliance with long term treaments in all conditions is often low. In the case of NTM lung disease where treatments can be toxic and where immediate symptomatic benefits are not always obvious, encouraging adherence can be a major challenge.101 Gastrointestinal side effects are the most common issue, particularly with macrolides. Hepatoxicity is common with rifampicin, macrolides, impenem and tigecycline among others. Aminoglycosides cause renal toxicity and hearing impairment. Patients should be warned to report tinnitus, hearing loss of vestibular symptoms, not only if using aminoglycosides but also macrolides.1,27 Peripheral neuropathy is an important adverse effect of linezolid and ethambutol. Visual acuity should be tested in patients receiving ethambutol.1,27 Rifampicin induces liver enzymes leading to enhanced metabolism of many drugs (including macrolides and fluoroquinolones, but also many other drugs such as oral contraceptives, with the result that woman of child bearing potential should use alternative contraception). The medications used by patients with NTM-PD should be carefully reviewed prior to commencing therapy with a view to identifying potential drug interactions.1,27
Antibiotic susceptibility testingWhether antibiotic susceptibility testing is helpful to guide NTM therapy is controversial.1,27 There are clear discrepencies between in vitro resistance and in vivo response for a number of antibiotics with the exception of macrolides and amikacin.102 For M. avium complex pulmonary disease a clear link between macrolide and amikacin resistance and clinical outcomes has been shown.102 Similarly for M. kansasii lung disease rifampicin susceptibility predicts clinical response.103,104 Thus MAC isolates should be tested for resistance to clarithromcyin and M. kansasii isolates should be tested for resistance to rifampicin and clarithromycin. Isolates resistant to rifampicin may be tested against secondary agents such as rifabutin, ethambutol, isoniazid, fluoroquinolones and amikacin.1,27
For the rapidly growing Mycobacteria such as M. abscessus complex susceptibility testing is typically performed for amikacin, cefoxitin, fluoroquinolones, macrolides, tetracyclines, imipenem, linezolid, trimethoprim-sulfamethoxazole and aminoglycosides. Prolonged incubation to 14 days is used to identify inducible macrolide resistance.27
New antimicrobial agents for NTM lung diseaseAmikacin is active against the majority of NTM species buy systemic administraation of amikacin is associated with typical aminoglycoside toxicities such as oto and nephrotoxicity.105 The development of inhaled amikacin for refractory NTM lung disease may therefore represent a significant advance.106 Data supporting the use of nebulised amikacin are from retrospective cohort studies, but randomised controlled trials have recently completed using liposomal amikacin. In the phase 2 study in which 44 patients received inhaled amikacin and 45 received placebo in addition to a multidrug regimen for 84 days, the primary endpoint of change in semi-quantiative NTM culture was not met (p=0.072) but there was an increase in sputum clearance of NTM which is a more clinically relevant end-point (32% vs 9%, p=0.006).107
Linezolid has been recently repurposed from the treatment of Staphylococcal skin and soft tissue infections to the treatment of MDR-TB and also has activitiy against some NTM species.108–110 The long term safety is a major limitation as the drug is associated with peripheral neuropathy, haematological toxicity and other adverse effects.108 Winthrop and colleagues recently described a series of 102 patients with NTM-PD treated with linezolid. 45% developed adverse effects, with peripheral neuropathy being most common (24%), along with gastrointestinal upset (9%), anaemia (8%) and thrombocytopenia (6%). 87% of those developing adverse effects had to stop therapy.108 Tigecycline similarly has good in vitro activity against MABC but its use is limitated due to nausea/vomiing and other side effects.111
Clofaziimine has activity against MDR-TB infections and has recently been proposed as a potential drug in NTM infections.112–114 It is administered orally, can be well tolerated in MDR-TB and is active against both slow growing and rapid growing NTMs. Bedaquiline is another oral drug used successfully in the treatment of MDR-TB which has been reported in small series to be successfully used as a salvage therapy.115–118 Major concerns including QT prolongation have been reported although recent data in TB suggest this concern may be less severe than previously thought.118
NTM disease in real-lifeAdherence to clinical guidelines for pulmonary NTM is suboptimal to say the least. A recent multinational survey in the EU and Japan which included 1429 NTM-PD cases found remarkable divergence from practice guidelines. Only 16.9% of MAC-PD patients received the recommended regimen of rifampicin/ethambutol and a macrolide for greater than 6 months.119 There was also large variation in guideline compliance rates between countries. This suggests an urgent need to educate physicians about evidence-based NTM treatment and to raise awareness of the disease.
SurgeryThe poor response rates of standard chemotherapy for most NTM-PD cases means that surgery should be considered in any case where it is feasible.1,27,120 This is particularly the case in patients with refractory disease or where extensive drug resistance is a problem. Surgery may take the form of complete resection of localised disease, or debulking of an area of severe disease which is thought to be driving the majority of symptoms.1,27 The other major indication for surgery is massive haemoptysis that cannot be controlled with medical therapy or bronchial artery embolisation. Case series show microbiological responses of 80–100% with surgery for NTM but post-operative complications are a significant problem with a post-operative mortality rate of up to 3%.120–122 Surgical management is best delivered by a multidisciplinary team incorporating physicians and surgeons experienced in the management of NTM-PD.
PrognosisA failure to achieve a microbiological response by 6 monhs of appropriate antimicrobial therapy and a failure to achieve sputum culture conversion after 12 months of appropriate therapy have been suggested as definitions of treatment failure or refractory NTM disease.1,27 Overall treatment success rates vary markedly in the literature, from 13% to 86% for MAC disease for example.26 A recent meta-analysis of 16 studies including 1462 patients receiving macrolide based regimens found a sustained culture conversion rate of 60%.123
Prognosis is related to the initial severity of disease, to the initial therapy receiving and to the clinical phenotype. Koh et al. recently described the outcomes of a large series of 481 patients with MAC-PD who received >12 months of antibiotic treatment. Patients without cavitation were more likely to have a favourable outcome (88%) compared to those with fibrocavitary (76%) or a mix of cavitary and bronchiectatic disease (78%). This study emphasised the importance of re-infection in this patient population as 29% were re-infected or relapsed following treatment, with genotyping showing 74% of cases were due to reinfection.24
Research prioritiesA recent USA based project identified a series of patient centred research priorities for NTM, including topics around transmission, diagnosis such as achiving more rapid diagnosis and developing algorithms to identify patients at risk.124 Other recommendations included better microbiological techniques that are more sensitive, and improved treatment options. Among the treatment recommendations were improved treatment for anxiety and depression, improved non-pharmacological treatments such as exercise and airway clearance and improved methods including possibly algorithms or prediction tools to identify patients who will require antibiotic treatment. The authors also recommended the development of a severity assessment algorithm similar to that already available in bronchiectasis, and the identification of biomarkers.124–127 For antibiotic therapy, there is a need to identify superior antibiotics, improved methods of delivering antibiotics, repurposed non-antibiotic drugs that may enhance mycobacterial clearance and regimens that can be given for a shorter duration.
This list may make it seem that no aspect of NTM disease has been fully optimised, and this is true. Nevertheless, substantial progress has been made in recent years in understanding the burden of disease, optimising diagnosis and implementing appropriate treatment.
The next 10 years will hopefully see breakthroughs in these areas that will start to make a major impact on the burden of NTM-PD
Conflicts of interestJD Chalmers reports research grants from Grifols, Insmed, Bayer Healthcare and Aradigm Corporation towards EMBARC, the European Bronchiectasis and NTM Registries.
The paper is part of the ERS/ALAT (and the ERS/SBPT collaborative projects (ERS: European Respiratory Society; ALAT: Latino-American Society of Respiratory Medicine): SBPT: Brazilian Society of Pulmonology).