Atherosclerosis is the main cause of cardiovascular disease (CVD), a major cause of mortality worldwide. A significant risk factor for atherosclerosis is obesity, which is also the main cause of Obstructive Sleep Apnoea (OSA).1 OSA, which causes nocturnal oxygen desaturation, is an independent risk factor for CVD, and it is also associated with atherosclerosis, although the mechanisms are not completely understood. In addition, there is mounting evidence that the gut microbiota (GM)has key immunity and metabolic functions that might play a role in the genesis of arteriosclerosis in patients with OSA.2,3 With the aim of demonstrating that OSA is an atherosclerotic disease from the start, Drager L et al., conducted a clinical study comparing two groups of patients: one of 15 young males with OSA without associated co-morbidities and another of 15 healthy volunteers (the control group), who took no preventive drugs for cardiovascular pathologies. This clinical trial showed that patients with OSA had early signs of atherosclerosis not detected in the control group without OSA, and that the level of vascular atherosclerosis was influenced by the severity of OSA.4 In addition, it was observed that extracellular vehicles (EVs) play an important role in the underlying inflammatory mechanisms of vascular arteriosclerosis. EVs are important intercellular mediators, responsible for both physiological and pathological processes, including chronic inflammation and neurodegenerative disorders.5 In accordance with a multidisciplinary and highly collaborative approach involving various scientific disciplines, Translational Medicine has perfected diagnostic tests based on EV levels (exosomes in particular) in bodily fluids and plasma, which can be regarded as early-onset biomarkers for atherosclerosis associated with OSA.6,7 A recent study demonstrated that serum levels of miRNA-149-3p and Hepcidin were high in OSA patients and were correlated with the severity of the disease and systemic inflammation. Levels of miR-149-3p and Hepcidin have a robust diagnostic value, and they have been shown to be predictive for OSA in the obese population.8 Furthermore, other studies9,10 have shown that the EVs released as part of the physio-pathological mechanisms involved in intermittent nocturnal hypoxia can activate coagulative processes and facilitate both thrombosis and the development of atherosclerotic cardiovascular disease (CVD). We believe that with the evolution of current techniques, miRNAs will become routine biomarkers in clinical practice, providing a valuable asset for precision medicine. The task now is to identify a panel of miRNAs that support a distinction between individuals affected by OSA (in its various phenotypes) and non-OSA individuals, as well as between high-risk and low-risk OSA patients, and to identify the various co-morbidities.
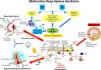
The Gut Microbiota (GM) is regarded as a systemic multi-organ microbial ecosystem with both immune and metabolic functions. Commensal and pathogenic bacteria coexist in homeostasis (eubiosis) within the host's intestinal epithelium and gastrointestinal immune system or GALT (Gut-Associated Lymphoid Tissue).11 However, an interruption of this homeostasis, referred to as dysbiosis, can be caused by various factors, such as psychological or physical stress, poor diet, use of antibiotics, age and chronic inflammatory processes, which can all cause dysfunction and damage communication between the intestine and the brain.12 A recent systematic review and meta-analysis showed that OSA is associated with gut barrier dysfunction based on PSG parameters.13 The microbiota is involved in many metabolic and inflammatory pathways that have been linked to the pathogenesis of Atherosclerotic Disease.14 Scientific data indicate that the GM plays a key role in arteriosclerosis and in the genesis of CVD by modulating chronic inflammation via the production of microbial metabolites.15-17 The connection between intestine and brain (BGMA Brain-Gut-Microbiome Axis) is believed to involve mainly three primary pathways, i.e. the immune system, the neuroendocrine system and the vagus nerve. It has recently been demonstrated that PAMPs (Pathogen-Associated Molecular Patterns) produced by intestinal bacteria are able to trigger cerebral inflammation.18 Moreover, there is supporting evidence19 showing that the GM is essential for physiological sleep maintenance and that it is possible to influence sleep and its stages (Non-REM and REM) by manipulating the intestinal microbiota, thus improving the patient's overall sleep, cognitive and behavioural sphere. The intestine and the brain communicate bidirectionally via the intestinal axis, which involves the autonomous nervous system, with the vagus nerve playing a pivotal role in the process. Its activation can reduce the intestinal inflammatory response by releasing acetylcholine, which interacts with immune cells to reduce the intensity of inflammation.20 It is also able to receive information regarding the osmotic concentration of the intestinal lumen, the quantity and quality of ingested nutrients including short-chain fatty acids (SCFAs) and the presence of metabolites produced by the intestinal microbiota (for example PAMPs). Ultimately, the vagus nerve can also provide neuroendocrine responses via its afferent fibres. The signals are detected and sent to the central nervous system (SNC), where in specific cerebral regions (including the amygdala, hypothalamus and cerebral cortex) they trigger the generation of specific hormonal, motor and behavioural responses.21 This immunomodulatory role of the vagus nerve can also affect the modulation of cerebral function. Given the importance of the microbiota in the modulation of neuronal and hormonal signalling, the concept of the “gut-brain axis” can be expanded to “microbiota-gut-brain axis” (seeFig. 1). The mechanisms and intercellular communication routes that govern the connections between the microbiota and the brain remain to be established. Targeting biomarkers of intestinal barrier dysfunction may offer new adjuvant therapeutic tools to reduce systematic inflammation in patients with OSA