Although the prevalence of severe asthma is not high (5–10% of patients), it is responsible for a large part of the overall disease burden and costs (50–60% of total costs), especially if the condition remains uncontrolled (which occurs in around 40% of cases).1 Currently, for patients without disease control or presenting frequent exacerbations despite optimal therapy, add-on treatments, traditionally long-acting anticholinergics, oral corticosteroids (OCS) or biologic agents (monoclonal antibodies) are recommended.2 Nonetheless, the long-term use of oral/systemic corticosteroids (CS) is significantly associated with adverse effects, acute and chronic complications that may decrease health-related quality of life and worsen prognosis, thus requiring additional monitoring and management. Conversely, target therapies (i.e., omalizumab, mepolizumab, reslizumab, benralizumab and more recently, dupilumab) have been developed grounded on the different phenotypes and endotypes of severe asthma, and are gradually reducing the reliance on OCS (i.e., greater specificity for achieving disease control by reducing the risk of exacerbations and requirements for rescue medication and OCS, with limited adverse events).3,4
In 2020, our research group performed a Delphi consensus in Portugal that showed a favorable perception among physicians for using biologic agents in severe asthma5; however, several questions including drugs availability, costs, patient eligibility and when to start therapy, remained to be clarified.6,7 This is especially important as therapeutic approaches can vary widely among clinical settings, which broadens the gap between real-world practices and intensifies the discussions on therapeutic optimization. Thus, with the goal of optimizing the use of systemic CS in adults with severe asthma in Portugal, including eligible and ineligible patients for biological therapy, we performed a nationwide consensus among pulmonology and immunoallergology experts.
This study was a 3-phase modified Delphi exercise consisting of a pre-round for developing the statements and two sequential rounds of anonymous questionnaires (1st and 2nd rounds) done online (May-July 2021). A total of 58 statements were developed by the scientific committee based on a literature search in PubMed using the keywords “severe asthma”, “corticosteroids” and “biologics” combined with the Boolean Operators AND and OR (n = 2.757 reviewed papers published between 2010 and 2020). These statements were grouped into three topics: (1) CS in severe asthma (n = 32 items); (2) CS in patients eligible for biological therapy (n = 17 items); (3) CS in patients not eligible for biological therapy (n = 9 items). A five-point Likert-type scale was used (1-‘strongly disagree’; 5-‘strongly agree’) to individually rate the statements in each round; consensus threshold was established as a percentage of agreement among participants (≥90% in the 1st round; ≥85% in the 2nd round). The level of consensus achieved by the participants was discussed by the scientific committee. Detailed methods have been previously published5; procedures followed standards for scientific research and were performed according to the Declaration of Helsinki. The scientific committee comprised six experts with experience in the treatment of severe asthma. The expert panel selected by the scientific committee consisted of 48 physicians (female:male 28:20; 26 pulmonologists and 22 immunoallergologists) with clinical and academic expertise in the management of severe asthma in Portugal (median H-index: 7.5 [IQR 2.75–13.25; minimum-maximum: 1–45], summing over 990 published articles indexed in PubMed), working in public or private institutions distributed at national level to capture regional specificities.
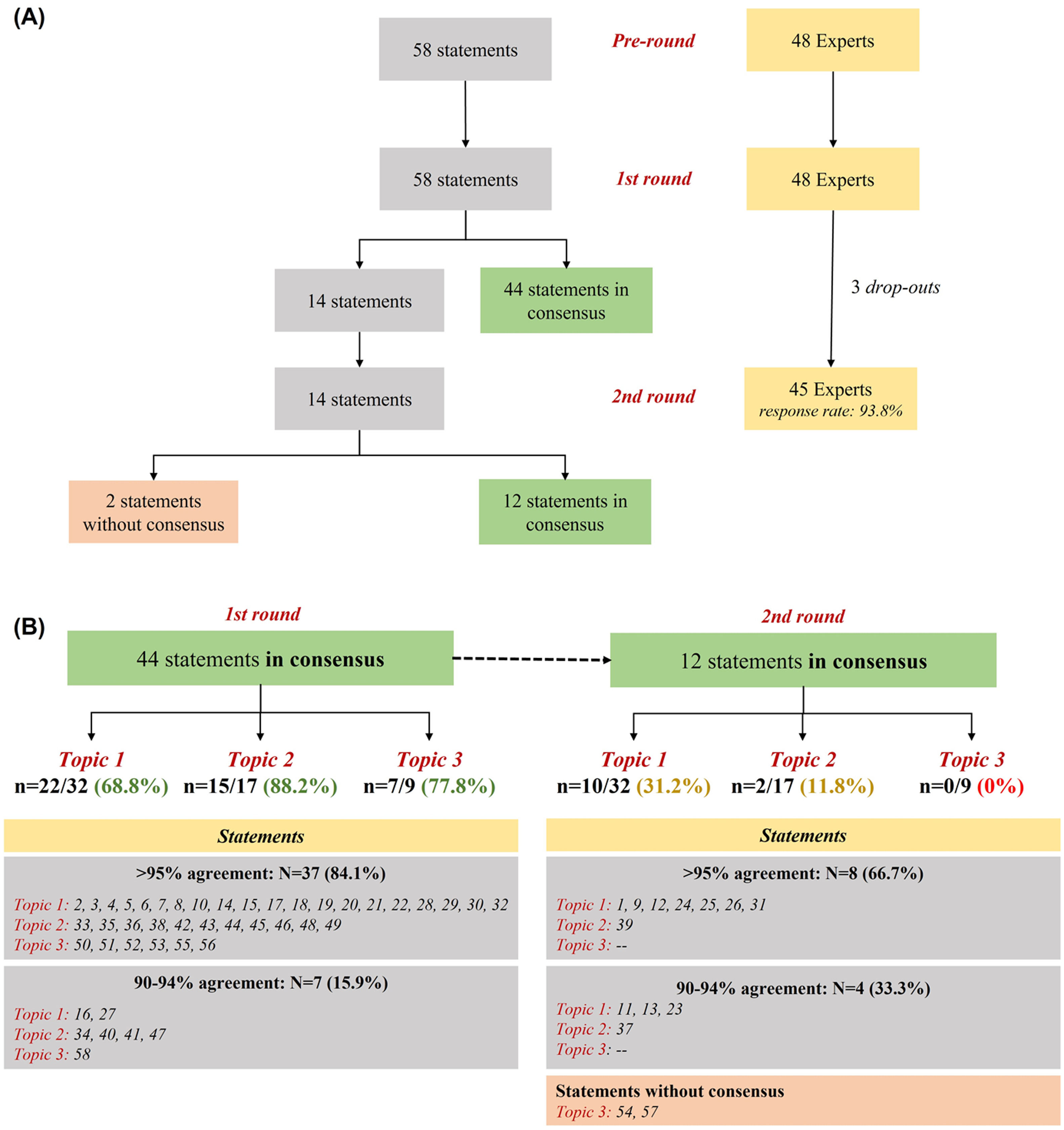
Overall, 45 experts participated in the study (93.8% response rate) (Fig. 1A). Around 75% (n = 44) of statements obtained positive consensus by the end of the 1st round. Most statements (n = 37; 84.1%) had a concordance over 95%, with seventeen of them (around 40%) presenting an agreement rate equal to 100%. Fourteen remaining items were iterated in the 2nd round, where 12 (85.7%) reached positive consensus (Fig. 1B). Table 1 summarize all rounds of consensus. These findings reinforce the conclusions from our previous Delphi study5 on the need for best practices in severe asthma management during the entire journey of the patient, including early specialist referral, phenotyping evaluation, risk factors/comorbidities assessment, therapeutic selection and rational use of OCS (e.g. patients’ eligibility, tailored tapering, early start of biologics) aiming at reducing or avoiding its related-risks (e.g. adverse events, toxicity), especially during chronic use. Conversely, by the end of the study, two statements (3.4%) [items 54 and 57 - both from Topic 3], were not consensual. In fact, statements from this topic focusing on the use of systemic CS in patients not eligible for biological therapy, were significantly less consensual compared to the other two topics (p = 0.02). One possible explanation for this is the lack of data for therapeutic decisions when biological therapy is unavailable.8
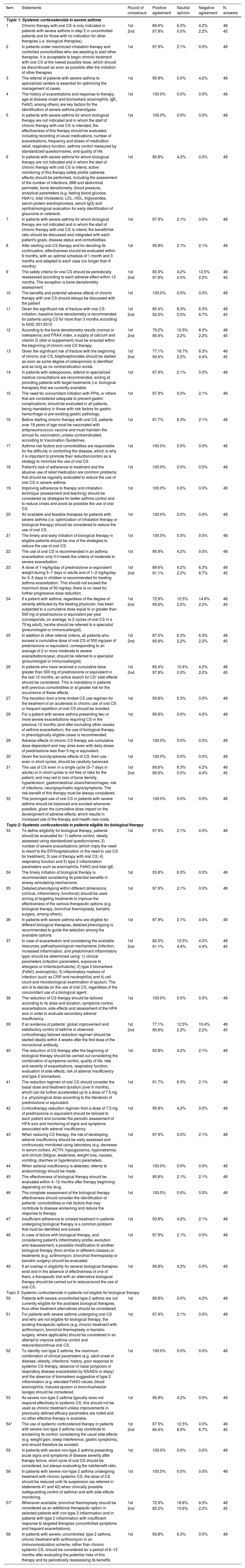
Results of the Delphi exercise.
| Item | Statements | Round of consensus | Positive agreement | Neutral opinion | Negative agreement | N. answers |
|---|---|---|---|---|---|---|
| Topic 1: Systemic corticosteroids in severe asthma | ||||||
| 1 | Chronic therapy with oral CS is only indicated in patients with severe asthma in step 5 in uncontrolled patients and for those with no indication for other therapies (i.e. biological therapies). | 1st | 89.6% | 6.3% | 4.2% | 48 |
| 2nd | 97.8% | 0.0% | 2.2% | 45 | ||
| 2 | In patients under maximized inhalation therapy and controlled comorbidities who are awaiting to start other therapies, it is acceptable to begin chronic treatment with oral CS at the lowest possible dose, which should be discontinued as soon as possible after the initiation of other therapies | 1st | 97.9% | 2.1% | 0.0% | 48 |
| 3 | The referral of patients with severe asthma to specialized centers is essential for optimizing the management of cases. | 1st | 95.8% | 0.0% | 4.2% | 48 |
| 4 | The history of exacerbations and response to therapy, age at disease onset and biomarkers (eosinophils, IgE, FeNO, among others) are key factors for the identification of severe asthma phenotypes. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 5 | In patients with severe asthma for whom biological therapy are not indicated and in whom the start of chronic therapy with oral CS is intended, the effectiveness of this therapy should be evaluated, including recording of usual medications, number of exacerbations, frequency and doses of medication relief, respiratory function, asthma control measured by standardized questionnaires, and quality of life. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 6 | In patients with severe asthma for whom biological therapy are not indicated and in whom the start of chronic therapy with oral CS is intend, active monitoring of this therapy safety profile (adverse effects) should be performed, including the assessment of the number of infections, BMI and abdominal perimeter, bone densitometry, blood pressure, analytical parameters (e.g. fasting blood glucose, HbA1c, total cholesterol, LDL, HDL, triglycerides, serum protein electrophoresis, serum IgG) and ophthalmological evaluation for early identification of glaucoma or cataracts. | 1st | 95.8% | 4.2% | 0.0% | 48 |
| 7 | In patients with severe asthma for whom biological therapy are not indicated and in whom the start of chronic therapy with oral CS is intend, the benefit/risk ratio should be discussed and integrated with each patient's goals, disease status and comorbidities. | 1st | 97.9% | 2.1% | 0.0% | 48 |
| 8 | After starting oral CS therapy and for deciding its continuation, effectiveness should be evaluated within 6 months, with an optimal schedule of 1 month and 3 months and adapted to each case (no longer than 6 months). | 1st | 95.8% | 2.1% | 2.1% | 48 |
| 9 | The safety criteria for oral CS should be periodically reassessed according to each adverse effect within 12 months. The exception is bone densitometry assessment. | 1st | 83.3% | 4.2% | 12.5% | 48 |
| 2nd | 97.8% | 0.0% | 2.2% | 45 | ||
| 10 | The benefits and potential adverse effects of chronic therapy with oral CS should always be discussed with the patient | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 11 | Given the significant risk of fracture with oral CS initiation, baseline bone densitometry is recommended for patients using CS for more than 3 months according to NOC 001/2010 | 1st | 85.4% | 8.3% | 6.3% | 48 |
| 2nd | 93.3% | 0.0% | 6.7% | 45 | ||
| 12 | According to the bone densitometry results (normal or osteopenia) and FRAX index, a supply of calcium and vitamin D (diet or supplement) must be ensured within the beginning of chronic oral CS therapy. | 1st | 79.2% | 12.5% | 8.3% | 48 |
| 2nd | 95.6% | 2.2% | 2.2% | 45 | ||
| 13 | Given the significant risk of fracture with the beginning of chronic oral CS, bisphosphonates should be started as soon as some degree of osteoporosis is identified and as long as no contraindication exists. | 1st | 77.1% | 16.7% | 6.3% | 48 |
| 2nd | 93.4% | 2.2% | 4.4% | 45 | ||
| 14 | In patients with osteoporosis, referral to specialized medical consultations are recommended, aiming at providing patients with target treatments (i.e. biological therapies) that are currently available. | 1st | 97.9% | 2.1% | 0.0% | 48 |
| 15 | The need for concomitant initiation with PPIs, or others that are considered adequate to prevent gastric complications, should be evaluated in all patients, being mandatory in those with risk factors for gastric hemorrhage or pre-existing gastric pathology. | 1st | 97.9% | 0.0% | 2.1% | 48 |
| 16 | Before starting chronic therapy with oral CS, patients over 18 years of age must be vaccinated with antipneumococcic vaccine and must maintain the annual flu vaccination, unless contraindicated, according to Vaccination Guidelines. | 1st | 91.7% | 6.3% | 2.1% | 48 |
| 17 | Asthma risk factors and comorbidities are responsible for the difficulty in controlling the disease, which is why it is important to promote their reduction/control as a strategy to minimize the use of oral CS. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 18 | Patient's lack of adherence to treatment and the abusive use of relief medication are common problems that should be regularly evaluated to reduce the use of oral CS in severe asthma. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 19 | Improving adherence to therapy and inhalation technique (assessment and teaching) should be considered as strategies for better asthma control and to reduce crises and avoid as possible the use of oral CS. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 20 | All available and feasible therapies for patients with severe asthma (i.e. optimization of inhalation therapy or biological therapy) should be considered to reduce the use of oral CS. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 21 | The timely and early initiation of biological therapy in eligible patients should be one of the strategies to reduce the use of oral CS. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 22 | The use of oral CS is recommended in an asthma exacerbation only if it meets the criteria of moderate to severe exacerbation. | 1st | 95.8% | 4.2% | 0.0% | 48 |
| 23 | A dose of 1 mg/kg/day of prednisolone or equivalent weight during 5–7 days in adults and of 1–2 mg/kg/day for 3–5 days in children is recommended for treating asthma exacerbation. This should not exceed the maximum dose of 50 mg/day; there is no need for further progressive dose reduction. | 1st | 89.6% | 4.2% | 6.3% | 48 |
| 2nd | 91.1% | 2.2% | 6.7% | 45 | ||
| 24 | If a patient with asthma, regardless of the degree of severity attributed by the treating physician, has been subjected to a cumulative dose equal to or greater than 500 mg of prednisolone or equivalent per year (corresponds, on average, to 2 cycles of oral CS in a 70 kg adult), he/she should be referred to a specialist (pneumologist or immunoallergist). | 1st | 72.9% | 12.5% | 14.6% | 48 |
| 2nd | 95.6% | 2.2% | 2.2% | 45 | ||
| 25 | In addition to other referral criteria, all patients who exceed a cumulative dose of oral CS of 500 mg/year of prednisolone or equivalent, corresponding to an average of 2 or more moderate to severe exacerbations/year, should be referred to a specialist (pneumologist or immunoallergist). | 1st | 87.5% | 6.3% | 6.3% | 48 |
| 2nd | 95.6% | 2.2% | 2.2% | 45 | ||
| 26 | In patients who have received a cumulative dose greater than 500 mg of prednisolone or equivalent in the last 12 months, an active search for CS’ side effects should be considered. This is mandatory in patients with previous comorbidities or at greater risk for the occurrence of these effects. | 1st | 85.4% | 10.4% | 4.2% | 48 |
| 2nd | 97.8% | 0.0% | 2.2% | 45 | ||
| 27 | The transition from a time-limited CS use regimen for the treatment of an acuteness to chronic use of oral CS or frequent repetition of oral CS should be avoided. | 1st | 93.8% | 6.3% | 0.0% | 48 |
| 28 | For a patient with severe asthma presenting two or more severe exacerbations requiring CS in the previous 12 months (and after excluding other causes of asthma exacerbation), the use of biological therapy in phenotypically eligible cases is recommended. | 1st | 95.8% | 0.0% | 4.2% | 48 |
| 29 | Adverse effects of chronic CS therapy are cumulative dose dependent and may arise even with daily doses of prednisolone less than 5 mg or equivalent. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 30 | Given the toxicity/adverse effects of CS, their use - even in short cycles, should be carefully balanced. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 31 | The use of CS even in a single cycle (5–7 days in adults) or in short cycles is not free of risks for the patient, and may led to loss of bone density, hypertension, gastrointestinal ulcers/hemorrhages, risk of infections, neuropsychiatric signs/symptoms. The risk-benefit of this therapy must be always considered. | 1st | 89.6% | 6.3% | 4.2% | 48 |
| 2nd | 95.6% | 0.0% | 4.4% | 45 | ||
| 32 | The prolonged use of oral CS in patients with severe asthma should be balanced and avoided whenever possible, given the cumulative dose impact on the development of adverse effects, which results in increased use of the therapy and health care costs. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| Topic 2: Systemic corticosteroids in patients eligible for biological therapy | ||||||
| 33 | To define eligibility for biological therapy, patients should be evaluated for: 1) asthma control, ideally assessed using standardized questionnaires; 2) number of severe exacerbations (which imply the need to resort to the ER/hospitalization or the need to use CS for treatment); 3) use of therapy with oral CS; 4) respiratory function and 5) type 2 inflammation parameters such as eosinophilia, FeNO and total IgE. | 1st | 97.9% | 2.1% | 0.0% | 48 |
| 34 | The timely initiation of biological therapy is recommended considering its potential benefits in airway remodeling mechanisms. | 1st | 93.8% | 6.3% | 0.0% | 48 |
| 35 | Detailed phenotyping within different dimensions (clinical, inflammatory, functional) should be used aiming at targeting treatments to improve the effectiveness of the various therapeutic options (e.g. biological therapy, bronchial thermoplasty, bariatric surgery, among others). | 1st | 97.9% | 2.1% | 0.0% | 48 |
| 36 | In patients with severe asthma who are eligible for different biological therapies, detailed phenotyping is recommended to guide the selection among the available options. | 1st | 97.9% | 2.1% | 0.0% | 48 |
| 37 | In case of exacerbation and considering the available resources, pathophysiological mechanisms (infection, increased inflammation, and predominant inflammatory type) should be determined using: 1) clinical parameters (infection parameters, exposure to allergens or irritants/pollutants); 2) type 2 biomarkers (FeNO, eosinophils); 3) inflammatory markers of infection (such as CRP and neutrophilia) and 4) cell count and microbiological examination of sputum. The aim is to decide on the use of oral CS, regardless of the concomitant use of a biological agent. | 1st | 83.3% | 12.5% | 4.2% | 48 |
| 2nd | 91.1% | 4.4% | 4.4% | 45 | ||
| 38 | The reduction of CS therapy should be tailored according to its dose and duration, symptoms control, exacerbations, side effects and assessment of the HPA axis in order to evaluate secondary adrenal insufficiency. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 39 | If an evidence of patients’ global improvement and satisfactory control of asthma is observed, corticotherapy tailored reduction regimen should be started ideally within 4 weeks after the first dose of the monoclonal antibody. | 1st | 77.1% | 12.5% | 10.4% | 48 |
| 2nd | 95.6% | 2.2% | 2.2% | 45 | ||
| 40 | The reduction of CS therapy after the beginning of biological therapy should be carried out considering the combination of symptoms control, quality of life, rate and severity of exacerbations, respiratory function, evaluation of side effects, risk of adrenal insufficiency and type 2 biomarkers. | 1st | 93.8% | 4.2% | 2.1% | 48 |
| 41 | The reduction regimen of oral CS should consider the basal dose and treatment duration (over 6 months), which can be further accelerated up to a dose of 7.5 mg (i.e. physiological dose according to the literature) of prednisolone or equivalent. | 1st | 91.7% | 6.3% | 2.1% | 48 |
| 42 | Corticotherapy reduction regimen from a dose of 7.5 mg of prednisolone or equivalent should be tailored to each patient and consider the periodic assessment of HPA axis and monitoring of signs and symptoms associated with adrenal insufficiency. | 1st | 95.8% | 4.2% | 0.0% | 48 |
| 43 | When reducing CS therapy, the risk of developing adrenal insufficiency should be early assessed and continuously monitored using laboratory (e.g. decrease in serum cortisol, ACTH, hypoglycemia, hyponatremia) and clinical (fatigue, weakness, weight loss, nausea, vomiting, diarrhea or hypotension) parameters. | 1st | 97.9% | 0.0% | 2.1% | 48 |
| 44 | When adrenal insufficiency is detected, referral to endocrinology should be made. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 45 | The effectiveness of biological therapy should be evaluated within 4–12 months after therapy beginning depending on the drug. | 1st | 95.8% | 2.1% | 2.1% | 48 |
| 46 | The complete assessment of the biological therapy effectiveness should consider the identification of patients’ comorbidities or risk factors that may contribute to disease worsening and reduce the response to therapy. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 47 | Insufficient adherence to inhaled treatment in patients undergoing biological therapy is a common problem that must be identified and solved. | 1st | 93.8% | 4.2% | 2.1% | 48 |
| 48 | In case of failure with biological therapy, and considering patient's inflammatory profile, evolution and reassessment, a possible modification to another biological therapy (from similar or different classes) or treatments (e.g. azithromycin, bronchial thermoplasty or bariatric surgery) should be evaluated. | 1st | 97.9% | 2.1% | 0.0% | 48 |
| 49 | If an overlap in eligibility for several biological therapies exist and in the absence of effectiveness of one of them, a therapeutic trial with an alternative biological therapy should be carried out to reduce/avoid the use of oral CS. | 1st | 95.8% | 4.2% | 0.0% | 48 |
| Topic 3: Systemic corticosteroids in patients not eligible for biological therapy | ||||||
| 50 | Patients with severe uncontrolled type 2 asthma are not currently eligible for the available biological therapies, thus other treatment alternatives should be considered. | 1st | 95.8% | 0.0% | 4.2% | 48 |
| 51 | For patients with severe asthma undergoing oral CS and who are not eligible for biological therapy, the existing therapeutic options (e.g. chronic treatment with azithromycin, bronchial thermoplasty or bariatric surgery, where applicable) should be considered in an attempt to improve asthma control and reduce/discontinue oral CS. | 1st | 97.9% | 2.1% | 0.0% | 48 |
| 52 | To identify non-type 2 asthma, the maximum combination of clinical parameters (e.g. adult onset of disease, obesity, infections’ history, poor response to systemic CS therapy, absence of nasal polyposis or respiratory disease exacerbated by NSAIDs or atopy) and the absence of biomarkers suggestive of type 2 inflammation (e.g. elevated FeNO values, blood eosinophilia, induced sputum or bronchoalveolar lavage) should be considered. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 53 | As severe non-type 2 asthma typically does not respond effectively to systemic CS, this should not be used as chronic treatment unless improvements in previously defined efficacy parameters are noted and no other effective therapy is available. | 1st | 95.8% | 4.2% | 0.0% | 48 |
| 54* | The use of systemic corticosteroid therapy in patients with severe non-type 2 asthma may contribute to worsening its control, considering the usual side effects (e.g. weight gain, sleep interference, gastric symptoms), and should therefore be avoided. | 1st | 87.5% | 12.5% | 0.0% | 48 |
| 2nd | 84.4% | 8.9% | 6.7% | 45 | ||
| 55 | In patients with severe non-type 2 asthma presenting acute signs and symptoms of disease severity after therapy failure, short cycle of oral CS should be considered, but always evaluating the risk/benefit ratio. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 56 | In patients with severe non-type 2 asthma undergoing treatment with chronic systemic CS, the dose of CS should be reduced until its suspension (as referred in statements 41 and 42) when clinically possible (safeguarding control of asthma) and with side effects monitoring. | 1st | 100.0% | 0.0% | 0.0% | 48 |
| 57* | Whenever available, bronchial thermoplasty should be considered as an additional therapeutic option in selected patients with non-type 2 inflammation and in patients with type 2 inflammation with insufficient response to targeted therapies (uncontrolled symptoms and frequent exacerbations). | 1st | 72.9% | 18.8% | 8.3% | 48 |
| 2nd | 82.2% | 15.6% | 2.2% | 45 | ||
| 58 | In patients with severe, uncontrolled, type 2 asthma, chronic treatment with azithromycin in an immunomodulation scheme, rather than chronic systemic CS, should be considered for a period of 6–12 months after evaluating the potential risks of this therapy and by periodically reassessing its benefits. | 1st | 93.8% | 6.3% | 0.0% | 48 |
One of the most relevant conclusions of our study was related to the overall consensus obtained on statement 38 (‘The reduction of CS therapy should be tailored according to its dose and duration, symptoms control, exacerbations, side effects and assessment of the HPA axis in order to evaluate secondary adrenal insufficiency’), which is aligned with the updated recommendations from GINA 2023 related to adrenal insufficiency in patients taking maintenance OCS or high dose ICS.2
Available biologic drugs for severe asthma usually target T2-high inflammatory pathways. Nonetheless, T2-low asthma is often a severe asthma subtype (associated with CS resistance) with limited treatment options (biologics are lacking for this indication) or scarce overall clinical evidence on their benefit or otherwise. The European Medicines Agency has recently approved tezepelumab (anti-TSLP, an alarmin) for the treatment of severe asthma without limiting a specific type of condition, although it is still not reimbursed in several countries; results regarding the use of this drug in patients previously non-eligible for biological therapy are promising.9 Yet, as mentioned in statement n. 53, physicians agreed that systemic CS should not be used in these patients as chronic treatment unless improvements in previously defined efficacy parameters are noted, and no other effective therapy is available. Conversely, no consensus regarding the use of CS in non-type 2 asthma patients or on the selection of alternatives for type 2 inflammation with insufficient response to targeted therapies were obtained (statement n. 54 ‘the use of systemic corticosteroid therapy in patients with severe non-type 2 asthma may contribute to worsening its control, considering the usual side effects (e.g. weight gain, sleep interference, gastric symptoms), and should therefore be avoided’). Although speculative, it is possible that some experts might still consider the use of systemic CS in this population due to the lack of current therapeutic options in the country for non-type 2 asthma. Current available GINA recommendations also consider the use of dupilumab for adults or adolescents requiring treatment with maintenance OCS, although available evidence for this indication is still scarce.2
Another controversial item among experts is related to the benefits of using bronchial thermoplasty for severe asthma (statement n. 57 ‘whenever available, bronchial thermoplasty should be considered as an additional therapeutic option in selected patients with non-type 2 inflammation and in patients with type 2 inflammation with insufficient response to targeted therapies (uncontrolled symptoms and frequent exacerbations)’). Yet, recent studies show that this technique may improve lung function and both asthma control and asthma quality of life scores in selected patients but with increased frequency of unscheduled doctor-visits and rescue courses of OCS and antibiotics.10
Although our study has some limitations related to the study design, relatively small sample, and large number of raw statements, it was strictly conducted according to a widely recognized method for achieving consensus and reflects the clinical practice challenges in severe asthma at a national level among selected key opinion leaders. These findings can support clinical decisions on oral/systematic CS use and tapering, adverse effects screening, and biologics initiation in severe asthma, while areas of that did not reach consensus, namely on the effects of CS for non-type 2 asthma and alternative approaches for non-responders to target therapies should be further investigated.
Both authors contributed equally to this manuscript.
The Portuguese ROSA Group is composed by: Ana Arrobas, Ana Luísa Ferreira, Ana Mafalda Van Zeller, Ana Mendes, Ana Todo Bom, Anna Sokolova, Beatriz Fernandes, Carlos Lopes, Catarina Teles Martins, Cecilia Pardal, Célia Costa, Claudia Chaves Loureiro, Claudia Pinto, Cristina Lopes Abreu, Emilia Faria, Estrella Alonso, Fernanda S. Tonin, Filipa Duarte-Ramos, Filipa Todo Bom, Frederico Regateiro, Inês Belchior, Ivone Fernandes, João Cordeiro Costa, João Marques, José Ferreira, José Manuel Silva, José Placido, Ligia Fernandes, Luís Amaral, Luis Taborda, Lurdes Ferreira, Manuel Branco Ferreira, Marta Drummond, Natacha Lopes, Natália André, Nuno Neuphart, Nuno Pires, Nuno Sousa, Paula Leiria Pinto, Paula Rosa, Pedro Martins, Ricardo Lima, Rita Boa Ventura, Rita Gerardo, Rodrigo Alves, Sofia Campina, Ulisses Brito.