Obstructive Sleep Apnoea Syndrome (OSAS) patients may develop hypertension and Positive Airway Pressure (PAP) is an effective treatment in blood pressure (BP) control.
ObjectivesAnalyse a hypertensive OSAS population with unexpected BP rise after PAP usage and verify correlations between BP rise, either with OSAS severity index or nocturnal ventilatory support compliance.
MethodsDescriptive, retrospective analysis of 30 patients with PAP treated OSA, for one year, on average, and with previous controlled hypertension, who developed a rise in BP, defined as augmentation of > 5mmHg in systolic (SBP) and/or diastolic BP (DBP), after PAP usage. Co-relational analysis of BP increase, with OSAS severity indexes and therapy compliance, using Pearson coefficient.
ResultsOf 508 consecutive patients followed in our Department, treated with nocturnal ventilatory support, 30 evolved with BP rise after initiating treatment (age 58±10.8 years; Apnoea-Hypopnoea Index [AHI], 46.1±18.68). After PAP usage, mean blood pressure (MBP), Systolic BP (SBP) and Diastolic BP (DBP) variation was 16±15mmHg, 20± 25mmHg and 6± 19.4mmHg, respectively. No patient showed significant BMI increase. Epworth Sleepiness Scale (ESS) value decreased 8.9 ± 5.48 points. MBP, SBP and DBP variations were not correlated with P90/P95, residual AHI, leaks or PAP compliance.
ConclusionsNo specific characteristics were identified in the group who developed a rise in BP with PAP usage. No correlations were found between rises in BP and OSAS severity indexes or PAP compliance. Neither BMI nor variation in wakefulness status explained the rise in BP. Studies relate polymorphisms of b1-adrenoreceptors with different BP responses to ventilatory support. More studies are needed to clarify the cause of this paradoxical response.
Doentes com síndrome de Apneia Obstrutiva do Sono (SAOS) podem desenvolver hipertensão arterial (HTA) sendo a pressão positiva na via aérea (PAP) um tratamento eficaz no controlo da pressão arterial (PA).
ObjectivosAnalisar uma população de doentes com SAOS que desenvolveu aumento inesperado da PA após o uso de PAP e verificar a existência de correlações entre o aumento da PA, quer com os índices de gravidade da SAOS, quer com a adesão ao tratamento com suporte ventilatório nocturno.
MétodosAnálise retrospectiva e descritiva de 30 doentes com SAOS e hipertensão previamente controlada, tratados com PAP, em média durante um ano, que desenvolveram um aumento da pressão arterial, definida como aumento de 5mmHg na Pressão Arterial Sistólica (PAS) e/ou Diastólica (PAD), após instituição de terapêutica PAP.
Análise correlativa do aumento da PA com os índices de gravidade da SAOS e com a adesão ao tratamento, utilizando o coeficiente de Pearson
ResultadosDe 508 doentes consecutivos seguidos no nosso Departamento, tratados com suporte ventilatório nocturno, 30 evoluíram com aumento de PA após o início do tratamento (idade 58 anos (SD 10.8), índice de Apneia-Hipopneia (IAH) 46.1 (SD 18.68)).
Após o uso de PAP, a variação da Pressão Arterial Média (PAM), da PAS e da PAD foi, respectivamente, de 16mmHg, 20mmHg e 6mmHg (SD: 15.0; 25.0; 19,4).
Nenhum doente mostrou aumento signifi cativo de Índice de Massa Corporal (IMC). O valor da Escala de Sonolência de Epworth (ESE) diminuiu 8,9 pontos (SD 5,48).
As variações de PAM, PAS e PAD não se relacionaram com P90/P95, IAH residual, fugas ou adesão a PAP.
ConclusõesNeste grupo que desenvolveu um aumento da PA com o uso de PAP não se identificaram características específicas. Não foram encontradas correlações entre os aumentos da PA, nem com os índices de gravidade de SAOS nem com a adesão à PAP. Nem a variação do IMC nem do wakefulness status explicaram o aumento da PA. Estudos têm relacionado polimorfi smos dos adrenoreceptores b1 com diferentes respostas da PA ao suporte ventilatório. Mais estudos são necessários a fim de clarificar a causa desta reacção paradoxal
Obstructive Sleep Apnoea Syndrome (OSAS) has a high prevalence. It affects 24 % of middle-aged men and 9 % of middle-aged women,1 and is symptomatic in 4–2 %, according to gender.2 Eighty five per cent of the clinically significant and treatable OSAS has never been diagnosed.3
Hypertension is also a great health problem that will affect 29.2 % of the adult population by the year 2025.4 In 2003, sleep apnoea was recognized as a common and identifiable cause of hypertension.5 About 30 % of patients with hypertension have OSAS and, 50 % of patients with OSAS have hypertension.3 OSAS may also be a frequent cause of drug -resistant hypertension.6
In normal sleep, compared to wakefulness, there is a 10–15 % decrease in blood pressure (BP), known as dipping phenomenon, associated with reduction in neural sympathetic traffic (in NREM sleep there is a lower BP, bradycardia and a reduction in cardiac output and systemic vascular resistance).7
Mechanisms of the pathophysiology of hypertension in OSAS are: increased sympathetic activity 3 (mostly caused by recurrent nocturnal hypoxia or hypercapnia, sleep fragmentation, or greater falls in pleural pressure due to the increased inspiratory effort8); baroreflex impairment;9 endothelial dysfunction; 10 vascular inflammation; 11 and atherosclerotic development.12
Several studies have demonstrated that Continuous Positive Airway Pressure (CPAP) is an effective treatment for hypertensive OSAS patients, although some patients are more difficult to control.3 Recent randomised controlled trials have shown that, compared to controls, CPAP treatment induces BP fall of 3.3mmHg, with a greater fall of 6.6mmHg in those on antihypertensive drugs.13
However, chronic effects of CPAP treatment are not so clear.14 Regarding the effects of antihypertensive drugs on OSAS, it has been reported that, none of the five most commonly used drugs (atenolol, amlodipina, enalapril, losartan and hydrochlorothiazide) showed an effect on overall BP and sleep architecture.3
Despite this evidence, we have noticed in our clinical practice that, some of our OSAS patients treated with nocturnal ventilatory support, with previously diagnosed and controlled hypertension, had a paradoxical increase in BP values.
ObjectivesThe main objective was to analyse general characteristics of an hypertensive OSAS population with BP rise after PAP usage (age; gender; body mass index (BMI); smoking habits, alcohol and caffeine consumption; comorbidities and therapeutics).
The second one was to verify whether there was any correlation between a rise in BP, either with OSAS severity index, or with nocturnal ventilatory support compliance.
Materials and methodsPopulationFrom a universe of 508 patients, diagnosed with OSA, and treated with nocturnal ventilatory support and followed in our Sleep Clinic (Autoadjusting PAP [APAP] or CPAP) for on average one year, we selected 30 patients (28 male and 2 female), who had hypertension which had been diagnosed and controlled and who developed a paradoxical BP reaction to ventilatory treatment, with rising values, measured regularly in each medical appointment in our clinic.
Sleep studyAll patients were diagnosed using an overnight cardio-respiratory domicilliary sleep study with a five-channel recording device (Alphascreen; Vyasis, Hoechberg-Germany). This device produces a computerized recording of variations in oronasal airflow (measured by nasal cannula), body position, wrist actimetry, pulse rate, arterial oxygen saturation (measured by finger pulse oximetry), thoracic and abdominal respiratory efforts. In all cases, sleep technicians carried out a manual analysis of the recordings, by counting apnoea (episodes of < 20 % of previous airflow with at least 10 seconds of duration) and hypopnoea episodes (episodes showing 20 to 50 % of the previous airflow, with at least 10 seconds of duration joined with a 4 % dip in oxygen saturation), dividing the total number of these episodes by the sleep time in hours, thus obtaining the manual apnoea-hypopnoea index.15
Blood pressureWe have defined a rise in BP as an augmentation of ≥ 5mmHg in systolic and/or diastolic BP, measured by manual sphygmomanometer.
Resting BP was measured in the sitting position, after 5-min rest, using a manual sphygmomanometer, and an appropriate sized cuff was fitted to the non-dominant arm. The first and last Korotkoff sounds were used to determine systolic and diastolic BP, respectively. We have registered the baseline values and those recorded on subsequent visits to our laboratory.
TreatmentAll subjects were under APAP (n = 27) or CPAP (n = 3), the latter titrated manually during an in-lab polysomnography, when APAP were not effective (AHI > 5/h under APAP), for home treatment. All had a minimum of 3 months effective treatment (> 70 % days; > 4h/night) with nocturnal ventilation, being 12.7 ± 7.3 the mean therapy months.
Ventilators in use were REM STAR Auto® (Respironics Inc., Murrysville, PA, USA).
The daytime sleepiness was evaluated with the Epworth Sleepiness Scale (ESS).
Total hours of CPAP usage were checked with software compatible with ventilators registries; mean daily CPAP use was calculated as total hours divided by number of days with CPAP at home.
Ventilation parameters (Maximum Pressure [PMax], Minimum Pressure [PMin] and Pressure at 90 or 95 % of the time [P90/95]) were registered, when on APAP, allowing the clinicians to verify the stability or not of the disease and the pressure required to treat respiratory events.
BP was controlled at baseline, in all enrolled patients, using: angiotensin converting enzyme inhibitors (ACEi) (32.3 %) (n = 10), CCA (29 %) (n = 9), thiazides and ARA-II (19.4 % each) (n = 6), β-blockers (12.9 %) (n = 4) and diuretics (9.7 %) (n = 3).
Six patients were treated with one antihypertensive drug; seven patients with two; three patients with three and two with more than three. Twelve patients were taking antihypertensive medication irregularly but had, nevertheless, blood pressure values within the normal range, at baseline evaluation.
The most frequent anti-hypertensive drugs association was an ACEi with a thiazide, with or without other anti-hypertensive drug.
In none of the cases was anti-hypertensive treatment changed during the reported follow-up.
Data analysisStatistical software (version 13.0; SPSS; Chicago, IL) was used for data processing and statistical analysis. Continuous variables were expressed as mean ± SD and qualitative variables were expressed as percentage.
The relationship between rises in BP, either with OSAS severity index, or with adhesion to nocturnal ventilatory support, was assessed by Pearson correlation.
ResultsEach patient was evaluated in several visits to our laboratory (n = 4.6 ± 2.1).
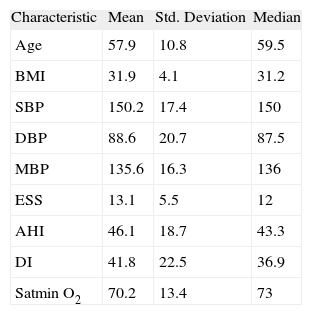
The studied population characteristics are depicted in Table 1.
Studied population characteristics (n = 30) at baseline
| Characteristic | Mean | Std. Deviation | Median |
| Age | 57.9 | 10.8 | 59.5 |
| BMI | 31.9 | 4.1 | 31.2 |
| SBP | 150.2 | 17.4 | 150 |
| DBP | 88.6 | 20.7 | 87.5 |
| MBP | 135.6 | 16.3 | 136 |
| ESS | 13.1 | 5.5 | 12 |
| AHI | 46.1 | 18.7 | 43.3 |
| DI | 41.8 | 22.5 | 36.9 |
| Satmin O2 | 70.2 | 13.4 | 73 |
AHI: Hypopnoea Apnoea Index; BMI: Body Mass Index; DBP: Diastolic Blood Pressure (mmHg); DI: dessaturation index; EES: Sleepiness Epworth Scale; MBP: Mean Blood Pressure (mmHg); Satmin 02: minimum 02 saturation (%); SBP: Systolic Blood Pressure (mmHg).
Seven patients presented moderate OSAS (mean AHI, 24.2 ± 3.25) and 24 severe OSAS (mean AHI 51.1 ± 16.57).
All subjects were under CPAP (n = 3) or APAP (n = 27) for home treatment. All had a minimum of 3 months effective treatment with nocturnal ventilation, 12.7 ± 7.3 were the mean months of therapy.
Regarding the habits of the studied population, 40.1 % had caffeine consumption, with an average 1.5 coffee/day (≈ 140mg of caffeine); 46.9 % presented moderate to heavy alcohol consumption; 31.3 % were smokers, with a mean pack/year of 38 and 12.5 % were ex-smokers.
The most frequently found comorbidities were: dislipidemia in 65.6 %, non-insulin dependent diabetes mellitus (NIDDM) in 18.7 %, hyperuricemia in 18.7 % and other cardiovascular pathology in 15.6 %, namely arrhythmias and left ventricular hypertrophy.
Regarding BP values, before ventilatory support, the mean value of SBP, DBP and mean blood pressure (MBP) were 150.2 ± 17.35mmHg; 88.6 ± 20.68mmHg and 135.6 ± 16.28mmHg, respectively.
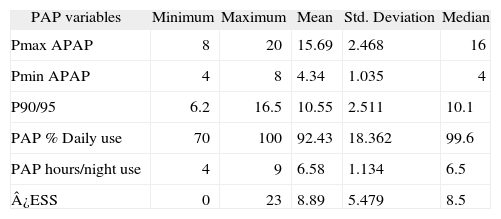
Nocturnal ventilatory support data is presented in Table 2.
PAP compliance (n = 30)
| PAP variables | Minimum | Maximum | Mean | Std. Deviation | Median |
| Pmax APAP | 8 | 20 | 15.69 | 2.468 | 16 |
| Pmin APAP | 4 | 8 | 4.34 | 1.035 | 4 |
| P90/95 | 6.2 | 16.5 | 10.55 | 2.511 | 10.1 |
| PAP % Daily use | 70 | 100 | 92.43 | 18.362 | 99.6 |
| PAP hours/night use | 4 | 9 | 6.58 | 1.134 | 6.5 |
| ¿ESS | 0 | 23 | 8.89 | 5.479 | 8.5 |
DEES: Sleepiness Epworth Scale variation pre- and post- treatment; PAP: Positive Airway Pressure; Pmax APAP: Maximum Pressure in Autoadjusting Positive Airway Pressure (cmH2O); PminAPAP: Minimum Pressure in Autoadjusting Positive Airway Pressure (cmH2O); P90/95: Pressure at 90 or 95 % of the time (cmH2O).
The leaks were at an elevated but acceptable level (30.6 ± 16.99L/min).
The mean value registered for the residual apnoea /hypopnoea index (AHI) was 4.6 ± 2.51.
We found a 6 % (30 patients in 508) prevalence of paradoxical BP response to PAP.
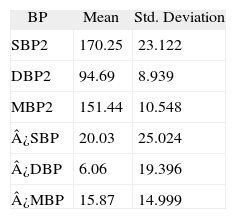
BP variation values are showed in Table 3.
Blood pressure variations with PAP usage (n = 30)
| BP | Mean | Std. Deviation |
| SBP2 | 170.25 | 23.122 |
| DBP2 | 94.69 | 8.939 |
| MBP2 | 151.44 | 10.548 |
| ¿SBP | 20.03 | 25.024 |
| ¿DBP | 6.06 | 19.396 |
| ¿MBP | 15.87 | 14.999 |
BP: Blood Pressure; DBP2: Diastolic Blood Pressure after Positive Airway Pressure usage (mmHg); ¿DBP: Diastolic Blood Pressure variation after Positive Airway Pressure usage (mmHg); ¿MBP: Mean Blood Pressure variation after Positive Airway Pressure (mmHg); ¿SBP: Systolic Blood Pressure variation after Positive Airway Pressure usage (mmHg); MBP2: mean Blood Pressure after Positive Airway Pressure usage (mmHg); SBP2: Systolic Blood Pressure after Positive Airway Pressure usage (mmHg).
Patients treated with β-blockers (in monotherapy or combined) had, on average, the lowest increase either in SBP (18mmHg) or in MBP (14mmHg) when compared with patients under other anti-hypertensive drugs. On the contrary those treated with diuretics (in monotherapy or combined) had the highest increase in SBP (51mmHg), DBP (19mmHg) and MBP (32mmHg). However, between groups, there was no statistic significance found for this means.
In relation to anti-hypertensive drugs used in the studied patients, BP was controlled prior to nocturnal ventilatory support (in monotherapy or combined) with: angiotensin converting enzyme inhibitors (ACEi) (30 %) (n = 9), CCA (26.7 %) (n = 8), thiazides and ARA-II (20 % each) (n = 6), β-blockers (13.3 %) (n = 4) and diuretics (10 %) (n = 3). Seven patients were treated with two antihypertensive drug; three patients with three and two patients with more than three.
The most frequent association was an ACEi with a thiazide, with or without another anti-hypertensive drug.
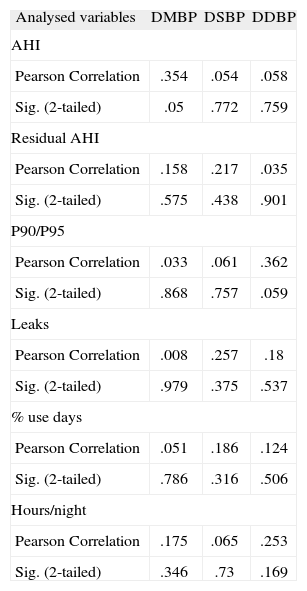
MBP, SBP and DBP variations were not correlated with OSAS severity indexes (AHI) or with nocturnal ventilatory support efficacy (considering residual AHI, P90 or P95, leaks) and compliance (considering % use days, hours/night). (Pearson's correlation coefficients are showed on Table 4).
Pearson correlation coefficient of MBP, SBP and DBP variations (after PAP usage) with residual AHI, P90 or P95, leaks, % use days, hours/night (n = 30)
| Analysed variables | DMBP | DSBP | DDBP |
| AHI | |||
| Pearson Correlation | .354 | .054 | .058 |
| Sig. (2-tailed) | .05 | .772 | .759 |
| Residual AHI | |||
| Pearson Correlation | .158 | .217 | .035 |
| Sig. (2-tailed) | .575 | .438 | .901 |
| P90/P95 | |||
| Pearson Correlation | .033 | .061 | .362 |
| Sig. (2-tailed) | .868 | .757 | .059 |
| Leaks | |||
| Pearson Correlation | .008 | .257 | .18 |
| Sig. (2-tailed) | .979 | .375 | .537 |
| % use days | |||
| Pearson Correlation | .051 | .186 | .124 |
| Sig. (2-tailed) | .786 | .316 | .506 |
| Hours/night | |||
| Pearson Correlation | .175 | .065 | .253 |
| Sig. (2-tailed) | .346 | .73 | .169 |
AHI: Hypopnoea/Apnoea index; ¿DBP: Diastolic Blood Pressure variation after Positive Airway Pressure usage (mmHg); ¿MBP: Mean Blood Pressure variation after Positive Airway Pressure (mmHg); ¿SBP: Systolic Blood Pressure variation after Positive Airway Pressure usage (mmHg); P90/95: Pressure at 90 or 95 % of the time (cmH2O).
The global features of our hypertensive OSAS population differed to that found in a recent metanalysis, 13 our population was older (mean age 57.9 vs 51.3), with more severe OSAS (mean AHI 46.1 vs 36.2) and with higher BP values (SBP - 150.2 vs 130.9 and DP - 88.6 vs 80.1).
Nowadays, the prevalence of hypertension is gender similar16 and prevalence of OSA is 3.2 men for each woman.17 We found in the studied population a greater predominance of male gender (93.5 %), much higher than expected.
The comorbidities found were those expected in these kinds of patients.18
Regarding the baseline BP values, and considering those found in a metanalysis (that included five studies with clinical BP evaluation, using the same manually inflated cuff as we did),14 mean SBP and mean DBP were 130.9 and 80.1mmHg, respectively, similar to those found in a 24h ambulatory BP monitoring (24h - ABPM) analysis.13 Judging by these results, the lack of a 24h-ABPM may not be a significant limitation in the present study.
The authors could not find a correlation between the OSAS severity indexes and the BP values but, on SBP and DBP, the values were not far from statistical significance. So, we can speculate that if the sample had been bigger the correlation could have existed.
Compliance to therapy was very good, with a mean daily use superior to that found to be cardiovascular protective 10,19,20 and a concordant improvement in subjective daytime sleepiness (ESS decreased 8.9 points). Also the treatment was effective as residual AHI was under 5/h.
We can state that the increases in BP observed in this population cannot be explained by under treatment, bad compliance or changes in anti-hypertensive drugs as they did not occur.
The data obtained for the final BP values showed a greater increase in SBP than DBP. We already knew that long-term CPAP treatment was more likely to re-establish the normal circadian dipping pattern of patients rather than influence the DBP change.9
Also, the lowest increase of BP was on patients treated with β-blockers. So, we may hypothesize that, in our group, there is a paradoxical response of the adrenergic sympathetic system to PAP. Also, the existing polymorphisms in the β1-adrenoceptor gene described by others 21 may be involved. Although in the Cardiovascular Health Study no effect of the Arg16Gly and Gln27Glu polymorphisms on BP was shown, the R389R polymorphism may have a functional role in the treatment response of OSAS patients.20 Nevertheless, in Borgel's study, the DBP of OSAS patients treated with CPAP for 6 months were reduced significantly only in Gly389 carriers.22
These results are not yet explained. Further studies are needed in this direction and our findings, despite the small sample size, of this paradoxical effect on BP, on PAP OSAS treated patients, may be of great value, revealing a need for a more profound search and investigation.
We would like to comment on limitations of this study: 24 BPM would probably be a more accurate method than manual punctual BP measurement but we used a number of determinations (n = 4.6), on different days, for a mean of 12.7 months of follow up to establish the paradoxical BP response to PAP, so the effects of a single measurement are diluted.
Also, we did not perform a polysomnography study with PAP usage in order to exclude the existence of arousals that could maintain activation of the sympathetic system during the night, apart from PAP therapy. However, we can speculate about the effectiveness of the PAP treatment with these patients, as they show a low residual AHI and a good clinical response shown by the improvement in ESS.
ConclusionsIn our specific population there was a high prevalence of paradoxical BP response to PAP in male gender.
No other specific characteristics were identified in this sub-group of patients with OSAS and hypertension and no correlation was established between BP variations, OSAS severity indexes or treatment compliance.
Patients under β-blockers showed a smaller increase in BP values when compared to the others.
Some studies relate genetic polymorphisms of β1-adrenoreceptors R389G with different responses to anti-hypertensive treatment in patients with OSAS and ventilatory support.
More studies are needed to clarify the cause of the paradoxical response of BP in these patients.
Conflict of interestAuthors declare that they don't have any conflict of interest.