AGXT gene codes for the enzyme alanine glyoxylate aminotransferase, which is involved in hepatic peroxisomal metabolism of platinum-based chemotherapeutic agents. The association of genetic variant AGXT rs34116584 on the clinical outcome and response to chemotherapy of patients with non-small cell lung cancer (NSCLC) remains to be established. Our aim was to evaluate the association of functional AGXT gene polymorphism in NSCLC progression, considering as primary and secondary endpoint, progression free survival (PFS) and overall survival (OS), respectively.
MethodsGenotyping of theAGXT rs34116584 genetic polymorphism was performed by mass spectrometry on 168 DNA samples from patients with NSCLC (stages IIIA-IVB). Univariate survival analysis included the study of Kaplan-Meier curves with the Log-Rank test, while Cox regression was used as a multivariate analysis.
ResultsMultivariate analysis showed shorter PFS for T carriers [HR=2.0, 95% CI, 1.4−3.0, p<0.0001] and shorter OS [HR=1.8, 95% CI, 1.1−3.0, p=0.017] globally, as well as in a subgroup of patients (n=144) treated with first line platinum-based chemotherapy [HR=2.0, 95% CI, 1.3–3.1, p=0.001] and [HR=1.8, 95% CI, 1.1–3.1, p=0.026], respectively.
ConclusionThis polymorphism seems to have an impact on NSCLC progression, opening new perspectives for its inclusion as a pharmacogenetic predictor of response to platinum-based chemotherapy.
Lung cancer is one of the most common malignancies worldwide and the most common cause of cancer deaths in the past few decades, with over one million subjects yearly diagnosed 1. The 5-year survival rate is the lowest compared with other frequent malignancies 2. Among all primary lung cancers, non-small cell lung cancer (NSCLC) represents approximately 85% of cases. The 5-year relative survival rate has been increasing over the last years, particularly due to progress in treatment over the years 3.
Although targeted therapies have redefined treatment options for patients with molecularly defined NSCLC (eg, epidermal growth factor receptor [EGFR]-mutant, anaplastic lymphoma kinase [ALK]-rearranged NSCLC), these therapies are ineffective in those whose tumours lack such genetic alterations, which comprise the majority of NSCLC patients 4.
Standard-of-care first-line chemotherapy for advanced NSCLC without actionable driver mutations or low expression of programmed death-ligand 1 (PD-L1) has historically been platinum-doublet, cisplatin or carboplatin, with or without maintenance therapy 5. Despite its wide acceptance and use, platinum-based chemotherapy presents poor clinical outcomes and efficacy varies across patients. Currently, the combination of immune checkpoint inhibitors with chemotherapy in advanced driver mutation-negative NSCLC and tumour PD-L1 expression under 50%, has replaced the regimen of only platinum-based chemotherapy in first line treatment 6.
Beyond clinical and pathologic features, genetic variation is also considered a factor associated with treatment efficacy and prognosis 7. Single-nucleotide polymorphisms (SNP), account for 90% of genetic polymorphisms, with some responsible for distinct molecular roles, contributing to inter-individual functional variability, correlating with relevant phenotypic variations in medicine 8. The AGXT gene codes for the enzyme alanine glyoxylate aminotransferase, localized in hepatic peroxisomes, which is known to participate in glyoxylate detoxification 9. Mutations in this gene have been reported to alter subcellular targeting and have been associated with type I primary hyperoxaluria 10. A polymorphism in AGXT gene (rs34116584) is responsible for a C>T substitution at locus +32 that results in Pro-Leu substitution located at codon 11 of exon 1 11. The amino acid substitution at position 11 creates a conformational change that is related to decreased activity 11. The polymorphism AGXT rs34116584 was shown to be associated with progression-free survival (PFS) in patients with metastatic colorectal cancer in response to oxaliplatin 12. Here, we sought to evaluate whether this genetic variant was associated with clinical outcomes in NSCLC patients, under the platinum-based chemotherapy regimen.
Material and methodsPopulationThis study comprises a retrospective cohort of histologically confirmed NSCLC patients (n=168), which were recruited between August 2017 and October 2018 from Coimbra University Hospital. Subjects with concomitant primary tumour in another organ were excluded. Clinical information was retrieved from clinical charts on pathological background, medications, stage, Eastern Cooperative Oncology Group performance status (ECOG PS), tumour mutational status, type of cancer treatment and disease progression/death. Targeted therapies were administered to carriers of genetic alterations in EGFR and ALK, whereas checkpoint inhibitors were used as salvage therapy. Information on chemotherapy-related febrile neutropenia (grade 3–4) in patients admitted to hospital stay was retrieved from clinical charts. The primary endpoint was progression-free survival (PFS) and the time-to-disease progression was calculated in months from the date of first line chemotherapy until the date of progression according to RECIST criteria. Overall survival (OS) was included as secondary endpoint, and the time-to-death was computed in months from the date of first line chemotherapy until the date of death/date of last visit. The research was reviewed and approved by the Coimbra University Hospital’s Ethical Committee (ref. 0111/CES) and by the Portuguese National Committee for data protection (number 2588/2017). Informed consent was obtained from each participant in agreement with the Helsinki Declaration.
AGXT genetic polymorphism and genotypingThe single nucleotide polymorphism included in the present study (AGXT rs34116584) was selected after reviewing public databases, in silico analysis and review of scientific literature to identify this functional polymorphism with minor allele frequency above 1% 8,10,11. Each patient donated a sample of blood (∼8mL) for research, collected to EDTA-Vacutainer tubes, at the same time of blood collection for routine analytic follow-up. The collected blood was separated into plasma and buffy coat and stored at −80°C until further analysis. DNA was isolated and purified from diluted buffy coats, using EZ1 BioRobot and EZ1 DNA Blood kit (QIAgen). AGXT rs34116584 was genotyped using the Sequenom Mass ARRAY matrix-assisted laser desorption/ionization time-of-flight mass spectrometry platform (Sequenom, San Diego, CA, USA). Primers were designed using semi-automated Assay Design 3.1 Software (Sequenom).
Statistical analysisStatistical analyses were performed on SPSS statistics software V.25.0 and P values below 0.05 were considered statistically significant. Continuous variables were depicted as average±standard deviation or median (interquartile range) according to departure from normality using Shapiro-Wilk test. Additive (CC vs. CT vs. TT), recessive (CC/CT vs. TT) and dominant (CC vs. CT/TT) genetic models were stratified according to wild type allele C. The time-to-outcome for AGXT genotypes was tested using Kaplan-Meier curves and Log-rank test in univariate and Cox proportional hazard model for multivariate analyses. The univariate empirical analyses included AGXT genetic models as well as other clinicopathological covariates. A p-value <0.05 was used as criteria for inclusion of a clinical variable in the multivariate Cox regression analysis, whereas the genetic model to include was determined using the likelihood ratio. The estimates of sample size, power, and effect size (regression coefficient) for survival analyses that use Cox proportional hazards models were conducted using STATA 16.0. It also reports the number of events (failures) required to be observed in the study. Sample size and number of events were calculated assuming alpha=0.05 and power>0.8. For both endpoints, the effect size was calculated from the resulting Hazard Ratio of AGXT variable in multivariate analysis. The minimal sample size for PFS was n=62 with an estimated number of events of n=50, whereas for OS, the calculated sample size was n=173 and the estimated number of events n=77.
ResultsThe clinicopathological characteristics of participating subjects are described in Table 1. The anatomical localization of distant metastases at diagnosis (n=94) was distributed as pleura and lung (62.8%), extra-thoracic (29.8%) and multiple (7.4%). Regarding mutational status, we observed that 8.3% of patients (n=14) had EGFR mutation (exon 19 deletions or exon 21 mutation), whereas 3.0% (n=5) had rearrangements in the gene encoding anaplastic lymphocyte kinase. Platinum-based doublet chemotherapy was administered to 85.7% of NSCLC patients, most frequently the cisplatin combination. Adjuvant chemotherapy was administered in twelve patients. In a subgroup of patients with chronic renal disease (n=24) the doublet chemotherapy with carboplatin was the first choice. Fifty-one patients underwent checkpoint inhibitors as second-, third- and fourth-line therapy. The median time-to-disease progression and the median time-to-death was 7.5 (CI 95%, 6.1–9.0) and 30.0 months (CI 95%, 16.9–43.2), respectively.
Clinical and oncological characteristics of the patients (N=168).
| Clinical Variables | |
|---|---|
| Age, Mean ± SD | 64.8±10.7 |
| Gender, N (%) | |
| Male | 124 (73.8%) |
| Female | 44 (26.2%) |
| Smoking history, N (%) | |
| No | 31(18.5%) |
| Smoker | 13 (7.7%) |
| Previous smoker | 68 (40.5%) |
| pTNM 8th edition, N (%) | |
| IIIA | 20 (11.9%) |
| IIIB | 33 (19.6%) |
| IIIC | 21 (12.5%) |
| IVA | 65 (38.7%) |
| IVB | 29 (17.3%) |
| ECOG performance status at diagnosis, N (%) | |
| 0 | 39 (23.2%) |
| 1 | 86 (51.2%) |
| 2 | 39 (23.2%) |
| 3 | 4 (2.4%) |
| 4 | 0 (0%) |
| Histology, N (%) | |
| Adenocarcinoma | 117 (69.9%) |
| Squamous cell carcinoma | 42 (25.9%) |
| Adenosquamous | 6 (3.6%) |
| Others | 3 (1.8%) |
| First line systemic therapy, N (%) | |
| Platinum-based doublet chemotherapy | 144 (85.7%) |
| Cisplatin | 121 (84.0% |
| Carboplatin | 23 (16.0%) |
| Targeted therapy | 24 (14.3%) |
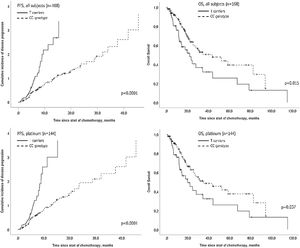
The AGXT rs34116584 genetic polymorphism distribution in this cohort of NSCLC patients was 71.7% C homozygous, 23.5% heterozygous and 4.8% T homozygous. Genotyping was successfully performed in 166 patients, with two missing genotyping. The median time-to-endpoint, hazard and survival univariate analyses of the empirical statistical procedure are depicted in Table 2. In the dominant genetic model, there was a significantly shorter PFS for T-allele carriers [5.4 months (CI 95% 4.3−6.4) versus 9.4 (CI 95%, 7.2−11.7), p<0.0001] and a shorter OS [22.2 months (CI 95% 13.6−30.8) versus 43.6 months (20.3−66.9), p=0.015] (Fig. 1). Notably, despite the AGXT rs34116584 T-carriers had shorter PFS than CC homozygous both in the subset of mutated (n=14, p=0.028) and wild-type (n=154, p<0.0001) EGFR, those AGXT carriers only presented shorter OS in wild-type (p=0.022) but not for mutated EGFR (p=0.692). Additionally, in a subset of patients with information on PD-L1 expression (n=98, 33.7% without and 66.3% with PD-L1 expression ≥1%), Kaplan-Meier plots with Log-Rank tests showed that T-carriers had shorter time-to-progression independently of PD-L1 positivity (p=0.010 and p=0.040, respectively).
Univariate analyses of AGXT rs34116584 and clinical variables with time-to-progression and time-to-death.
| Progression-free survival | Overall survival | ||||
|---|---|---|---|---|---|
| n | Median (95%CI) | P * | Median (95%CI) | P * | |
| Age | |||||
| <65.4 | 86 | 7.2 (5.8-8.7) | 43.6 (8.5-78.7) | ||
| >65.4 | 82 | 8.6 (5.1-12.1) | 0.541 | 23.6 (15.3-31.8) | 0.078 |
| Gender | |||||
| Male | 124 | 7.2 (5.3-9.1) | 28.1 (21.7-34.4) | ||
| Female | 44 | 8.9 (5.5-12.3) | 0.547 | 82.5 (15.1-150.0) | 0.102 |
| Histology | |||||
| Adenocarcinoma | 117 | 7.8 (5.7-9.9) | 44.0 (18.6-69.4) | ||
| Squamous cell | 5.7 (4.4-7.0) | 24.6 (17.0-32.2) | |||
| Others * | 429 | 9.5 (3.4-15.7) | 0.201 | 31.3 (19.3-43.4) | 0.069 |
| T | |||||
| 1 | 26 | 10.2 (4.1-16.3) | 30.0 (14.1-46.0) | ||
| 2 | 46 | 9.4 (4.0-14.9) | 67.4 (46.7-88.2) | ||
| 3 | 18 | 4.7 (1.4-8.1) | 26.9 (19.5-34.3) | ||
| 4 | 78 | 5.5 (4.0-7.1) | 0.008 | 25.4 (16.7-34.2) | 0.011 |
| N | |||||
| N0 | 13 | 9.0 (4.2-13.8) | – | ||
| N1 | 18 | 7.1 (2.3-12.0) | 82.5 (22.9-142.1) | ||
| N2 | 34 | 9.5 (2.8-16.2) | 26.9 (18.5-35.3) | ||
| N3 | 103 | 6.6 (4.6-8.6) | 0.151 | 31.3 (12.9-50.0) | 0.790 |
| M | |||||
| no | 74 | 9.6 (5.2-14.0) | 78.7 (53.0-104.4) | ||
| yes | 94 | 5.4 (4.5-6.2) | 0.003 | 22.2 (17.3-27.1) | <0.0001 |
| Type Therapy | |||||
| Surgery+CT | 12 | 20.5 (0.0-49.2) | – | ||
| CT | 125 | 6.6 (4.9-8.2) | 26.9 (21.0-33.0) | ||
| CT+RT | 31 | 8.9 (4.9-12.8) | 0.024 | 34.9 (7.2-62.5) | 0.188 |
| ECOG PS | |||||
| Good (0-1) | 125 | 8.0 (6.1-9.9) | 44.0 (21.2-66.8) | ||
| Poor (2-4) | 43 | 5.4 (2.7-8.1) | 0.171 | 12.9 (9.8-16.0) | <0.0001 |
| Systemic Therapy | |||||
| Platinum based | 144 | 6.2 (4.7-7.8) | 28.1 (20.0-36.2) | ||
| Target therapy | 24 | 13.3 (0.2-26.3) | 0.005 | - | 0.183 |
| AGXT rs34116584 | |||||
| Additive model | |||||
| CC | 119 | 9.4 (7.2-11.7) | 43.6 (20.3-66.9) | ||
| CT | 39 | 5.7 (5.0-6.4) | 17.8 (10.0-25.7) | ||
| TT | 8 | 4.0 (3.4-4.6) | <0.0001 | 24.6 (21.9-27.2) | 0.009 |
| Dominant model | |||||
| CC | 119 | 9.4 (7.2-11.7) | 43.6 (20.3-66.9) | ||
| CT/TT | 47 | 5.4 (4.3-6.4) | <0.0001 | 22.2 (13.6-30.8) | 0.015 |
| Recessive model | |||||
| CC/CT | 158 | 7.8 (6.3-9.2) | 31.3 (16.7-45.9) | ||
| TT | 8 | 4.0 (3.4-4.6) | 0.025 | 24.6 (21.9-27.2) | 0.615 |
CT, chemotherapy; ECOG PS, ECOG performance status; OS, overall survival; PFS, progression-free survival; RT, radiotherapy. * Log-Rank test. ** others: pleomorphic, combined squamous and adenocarcinoma. 95%CI, 95% confidence interval.
The statistically significant covariates from univariate analysis were included in a Cox proportional-hazards multivariate model. This data showed for AGXT T-carriers an increased risk for progression (HR=2.0; 95% CI, 1.4−3.0; p<0.0001) and for cancer-specific death (HR=1.8; 95% CI, 1.1−3.0; p=0.017), regardless of tumour size, distant metastasis at diagnosis, type of systemic therapy and type of treatment modality (Table 3). To test the hypothesis that AGXT rs34116584 was associated with the response to platinum-based chemotherapy, the analysis was conducted in the group of patients treated with first line platinum-based doublet chemotherapy (n=144). In this subgroup, there were no identifiable actionable driver mutations at the diagnosis. Univariate analysis showed longer PFS in C homozygous (median 8.6, CI 95%, 6.1–11.1 months) in comparison with T-carriers (median 5.1, CI 95%, 4.2–6.0 months) (p<0.0001) (Fig. 1). Concordantly, the time-to-death was also longer in CC (median 34.9, CI 95%, 12.1–57.6 months) compared to T-carriers (median 19.8, CI 95%, 8.9–30.7 months) (p=0.037) (Fig. 1). On multivariate analysis T-carriers had higher risk for disease progression (HR=2.0, 95% CI, 1.3–3.1, p=0.001) independently of relevant clinicopathological covariates. In platinum-treated patients, those with febrile neutropenia (n=20) exhibited more frequently the T-allele compared to non-febrile neutropenia (35% versus 29%, respectively), despite the lack of association of the SNP with myelotoxicity (OR=1.34, 95% CI, 0.49–3.64, p=0.566).
Multivariate Cox regression including only the significant covariates after empirical analysis, for PFS and OS.
| Progression-free survival | Overall survival | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| cT (TNM) | ||||
| T1 | Referent | Referent | ||
| T2 | 1.6 (0.9-2.8) | 0.131 | 0.6 (0.3-1.4) | 0.278 |
| T3 | 2.2 (1.1-4.6) | 0.026 | 0.9 (0.4-2.2) | 0.856 |
| T4 | 2.1 (1.2-3.7) | 0.007 | 1.6 (0.8-3.1) | 0.159 |
| Distant metastasis | ||||
| No | Referent | Referent | ||
| Yes | 1.6 (1.5-2.3) | 0.010 | 2.1 (1.3-3.7) | 0.005 |
| Systemic Therapy | ||||
| Platinum | referent | – | ||
| Target therapy | 0.4 (0.2-0.8) | 0.003 | – | – |
| ECOG PS | ||||
| Good (0-1) | – | Referent | ||
| Poor (2-4) | – | – | 2.3 (1.4-3.7) | 0.001 |
| Type of therapy | ||||
| Surgery+CT | Referent | – | ||
| CT | 2.7 (1.1-6.7) | 0.026 | – | |
| CT+RT | 2.8 (1.1-7.0) | 0.027 | – | – |
| AGXT rs34116584 | ||||
| Dominant model | ||||
| CC | Referent | Referent | ||
| CT/TT | 2.0 (1.4-3.0) | <0.0001 | 1.8 (1.1-3.0) | 0.017 |
CT, chemotherapy; ECOG PS, ECOG performance status; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; 95%CI, 95% confidence interval
In the past, advances in genetic knowledge about lung cancer mutational landscape, together with development of targeted therapies, led to a paradigm shift in the treatment of NSCLC. Nevertheless, platinum-containing regimens remain the appropriate treatment for most patients 13. Clinical management of resistance or toxicity to chemotherapy in NSCLC patients would benefit from the identification of predictive and prognostic molecular biomarkers, including functional genetic polymorphisms.
The AGXT gene, located in chromosome 2q37.3 region, encodes the alanine-glyoxylate aminotransferase, whose activity is largely confined to peroxisomes in the liver 14. This enzyme catalyses the transamination between L-alanine and glyoxylate to produce pyruvate and glycine using pyridoxal 5′-phosphate as cofactor 15. A missense genetic variant (AGXT rs34116584), with a proline-to-leucine substitution located at codon 11 of exon 1, occurs with a frequency of 15–20% in European and North American population 11. This polymorphism was primarily studied in primary hyperoxaluria type I 16–18. A recent report explored its role in cancer, showing an association with disease progression and death in metastatic colon cancer patients treated with oxaliplatin 12. Reports are sparse concerning the association of this SNP with cancer and have never been explored in lung cancer patients.
Herein, the AGXT-rs34116584 genetic polymorphism was analysed in locally advanced/metastatic NSCLC patients, using as outcomes the PFS and OS. Multivariate analyses revealed an independent increased risk for disease progression and for death in AGXT rs34116584 T-carriers, after adjustment for tumour size, distant metastasis, ECOG PS, treatment modality or systemic therapy. Previous molecular in vitro studies showed that the C-to-T substitution results in an amino acid modification at position 11 and creates a conformational alteration that ultimately leads to a significant decrease in alanine-glyoxylate aminotransferase´s activity and subsequent accumulation of oxalate 19,20. Both oxalate and glyoxylate generate reactive oxygen species (ROS) 21,22, which have been associated with increased mutational burden, tumour progression and dissemination 23. Since T-allele carriers have higher levels of oxalate 24 and consequently are prone to increased ROS production, the worst prognosis described for TT/TC might be an oxidative stress-mediated deregulation induced by AGXT rs34116584 SNP. This effect might be exponentiated upon exposure to hypoxia and oxidative stress causing DNA damage, or during concomitant administration to cytotoxic therapies 25.
Furthermore, a significantly shorter time-to-disease progression was found for T-allele carriers independent of EGFR mutational status, although no relation was observed with OS for subjects with EGFR tumour mutation. These findings could be aligned with a minor clinical relevance for AGXT rs34116584 SNP in comparison to EGFR mutation status that impacts a longer-term endpoint. Notably, tyrosine kinase inhibitors (TKIs) improve survival in NSCLC patients with EGFR mutation 26, modifying the natural history of disease, and possibly impacting the association of the genetic polymorphism.
In patients under first line platinum-based doublets, we verified that T-allele carriers had shorter PFS and OS; regardless of tumour size, distant metastasis, ECOG PS and treatment modality. These well-established prognostic covariates, were shown to influence NSCLC clinical outcomes 27. Here, the AGXT rs34116584 association with response to platinum-based chemotherapy remained significant, despite adjustment for these factors, suggesting that this SNP might add significant information to traditional clinical predictive and prognostic factors. The AGXT rs34116584 C>T substitution, induces a decrease of alanine-glyoxylate aminotransferase activity and is responsible for the mistargeting of the enzyme from the peroxisomes to the mitochondria, where the enzyme cannot work properly 10. These changes were predicted to have significant effects in oxalate synthesis and excretion, and the deposition of insoluble calcium oxalate in the kidney and urinary tract 28, which could be associated with increased toxicity and lesser efficacy of platinum based chemotherapy.
Moreover, cisplatin causes a number of significant side effects including nausea and vomiting, neutropenia, ototoxicity, neurotoxicity, and renal function impairment 29. Despite efforts to identify genetic predictors of the effectiveness and toxicity of cytotoxic therapies, up to now there are no robust data that can be used in clinical practice to guide the best subgroup of patients to receive cisplatin 29. Although carboplatin induces nephrotoxicity to a lesser extent, it induces more myelotoxicity 30. No association was found in our study for the AGXT rs34116584 SNP with febrile neutropenia, although the low number of subjects included in this analysis limits its conclusions.
To the best of our knowledge, this is the first report describing the prognostic impact of functional AGXT polymorphism in lung cancer patients. As such, further studies in larger independent populations are required to confirm these results. Despite inherent size limitations, in this study patients were recruited from a homogeneous cohort, the analysed SNP was selected based on functional biological relevance, and the study design and statistics accounted for important risk factors in NSCLC.
ConslusionThe functional impact of the AGXT rs34116584 SNP in decreasing the peroxisomal activity of the enzyme alanine glyoxylate aminotransferase influence oxalate accumulation. This effect might have an influence in platinum metabolization, with impact on toxicity and tumour aggressiveness, being associated with worse prognosis. This polymorphism seems to have an impact on NSCLC progression, opening new perspectives for its inclusion as a biomarker or as a pharmacogenetic predictor of response to platinum-based chemotherapy.
FundingMJ Catarata was supported by the Portuguese Pulmonology Society.
Ethics approvalThis project has been reviewed and approved by Coimbra University Hospital’s Ethical Committee (reference number 0111/CES; date of approval: 27th July 2017) and was also approved by the National Committee for data protection (number 2588/2017; date of approval: 6th March 2017).
Conflicts of interestAll authors declare that they have no conflict of interest.
The authors would like to acknowledge the lab technician’s Dr Elisabete Camilo, Dr Isabel Marques and Dr Andreia Coelho for their invaluable support for DNA extraction.