Cough capacity derangement is associated with a high risk of pulmonary complications in amyotrophic lateral sclerosis patients when cough assistance is not routinely performed at home. The primary aim of this study was to evaluate the feasibility of a long-term home based daily self-monitoring cough capacity.
MethodsEighteen subjects were enrolled in a 9-month study at home. Changes in peak cough expiratory flow, oxygen saturation, respiratory discomfort and incidence of respiratory deterioration events were evaluated. In subjects presenting respiratory deterioration events, decline in the abovementioned respiratory variables was evaluated (#NCT00613899).
ResultsDuring an average follow-up of 125±102 days, a total of 1175 measures were performed on 12 subjects. Mean compliance to proposed evaluations was 37±32% which worsened over time. Peak cough expiratory flow decreased by 15.08±32.43L/min monthly. Five subjects reported 6 episodes of respiratory deterioration events, after a mean period of 136±108 days. They had poor respiratory function and more years of disease. There was no difference in peak cough expiratory flow and its decline whether subjects presented respiratory deterioration events or not. In 4 subjects the respiratory discomfort score significantly worsened after respiratory deterioration events from 3.0±1.41 to 4.25±1.71.
ConclusionDaily self-monitoring of peak cough expiratory flow, oxygen saturation and respiratory discomfort seems difficult to obtain because of poor adherence to measures; this protocol does not seem to add anything to current practice of advising on clinical derangements. Confirmatory larger studies are necessary.
A disfunção na capacidade de tosse está associada a um elevado risco de complicações pulmonares nos doentes com esclerose lateral amiotrófica, quando a sua monitorização não é realizada rotineiramente no domicílio. O objetivo principal deste estudo foi avaliar a viabilidade de uma automonitorização domiciliária diária da capacidade da tosse, a longo prazo.
MétodosDezoito doentes foram incluídos num estudo com duração de 9 meses, realizado no domicílio. Foram avaliadas as alterações do débito expiratório máximo da tosse, a saturação de oxigénio, o desconforto respiratório e a incidência de eventos de deterioração respiratória. Em doentes que apresentavam eventos de deterioração respiratória, foi avaliada a diminuição nas variáveis respiratórias supracitadas (#NCT00613899).
ResultadosDurante um acompanhamento médio de 125±102 dias, foram realizadas um total de 1.175 medições em 12 doentes. A média de cumprimento para as avaliações propostas foi de 37±32%, e piorou ao longo do tempo. O débito expiratório máximo da tosse diminuíu em 15,08±32,43L/min mensalmente. Cinco doentes relataram 6 episódios de eventos de deterioração respiratória, após um período médio de 136±108 dias. Tinham uma função respiratória mais alterada e mais anos de doença. Não existia diferença no débito expiratório máximo da tosse e na sua diminuição, quer os sujeitos apresentassem eventos de deterioração respiratória ou não. Em 4 doentes o resultado de desconforto respiratório piorou significativamente após os eventos de deterioração respiratória, de 3,0±1,41 para 4,25±1,71.
ConclusãoA auto monitorização diária diária do débito expiratório máximo da tosse, da saturação de oxigénio e do desconforto respiratório parecem difíceis de obter devido à fraca adesão a sua determinação; este protocolo parece nada acrescentar à prática atual de aconselhamento sobre os distúrbios clínicos. É no entanto necessária a confirmação deste resultado em estudos posteriores com amostras de maior dimensão.
Deterioration of respiratory function is a critical factor in amyotrophic lateral sclerosis (ALS).1 Respiratory tract infections (RTIs) are the principal causes of morbidity and mortality.2 Low level of peak cough expiratory flow (PCEF) is associated with a high risk for pulmonary complications during RTIs,3–5 for hospisalization,6 and is also considered an indicator for spontaneous cough effectiveness during an acute RTI.6–8 PCEF reflects the capacity to expulse debris from the airways (cough efficacy) and values less than 160L/min are associated with extubation failure.9
After RTIs, subjects with neuromuscular diseases have a slow recovery of clinical, functional and oxygenation parameters.3
Studies have also demonstrated the importance of using specific cough assistance techniques9–13 in order to avoid rehospitalisation.4,11,14–18
Although easily evaluable, PCEF is not routinely done at home in subjects with ALS.
The primary aim of this study was to evaluate, in subjects with non-bulbar ALS, the feasibility of a long-term (9 months), home-based, comprehensive protocol involving daily self-monitoring for cough capacity. Changes in objective (PCEF and SpO2) and subjective (respiratory discomfort [RD]) respiratory variables, occurrence of respiratory deterioration events (RDEs) and influence of baseline PCEF and its decline during time on RDEs were evaluated as secondary outcomes.
MethodsSubjectsSubjects with diagnosis of ALS according to the El Escorial criteria,19 admitted to the Rehabilitation Respiratory Division of Fondazione S. Maugeri – Lumezzane (BS), were considered for this study. Inclusion criteria were: (1) ALS functional rating scale (ALS-FRS-R) score<35, (2) non-bulbar impairment at first presentation defined by clinical presentation and a PCF/PEF (peak espiratory flow) ratio>1, (3) PCEF<450L/m, (4) NIV prescription at home. Criteria for starting NIV were daytime hypercapnia, sleep-related hypoxemia, and decrease of vital capacity below 50% predicted.20 Exclusion criteria were refusal, tracheostomy, no caregiver availability, dementia, bulbar patients. The study was approved by the Technical and Scientific Committee of our Institute. All subjects gave informed consent.
MeasuresAt baseline the following tests or evaluations were carried out: (a) anthropometric characteristics, (b) ALS-FRS-R score, (c) respiratory function (FEV1, FVC, FEV1/FVC, VC, MIP, MEP) according to Quanier predictive indices,21 (d) arterial blood gases (ABG), (e) mechanical ventilation use,22 (f) PCEF measured at rest using a peak flow meter (Mini-Wright, standard range peak flow meter Clement Clarke International, UK) connected to a face mask (Ultraseal, Ambu A/S DK-2750 Ballerup Denmark). The patients will have to be kept in sitting and asked to cough as forcibly as possible (an unassisted cough manoeuvre). The maximum observed flows in four or five attempts were recorded.23
All subjects could generate peak cough flows because, at that time, they were able to close the glottis, to air stack. Socio-demographic characteristics of caregiver and hours of care/day were also recorded.
ProtocolSubjects received a pulse oximeter (NONIN Onyx® 9500 Fingertip Pulse Oximeter, Nonin Medical, INC. Plymouth, MN, USA) a peak flow meter with mask, a Borg scale sheet, and a clinical diary to be filled in. None of the subjects received mechanical cough assistance devices at home. During their stay at home, subjects were informed that they would receive telephonic support from a dedicated physiotherapist (PT) during working hours on a bi-weekly basis. At home, the patient/caregiver was requested, in the early morning and nocturnal application of NIV on a daily basis to measure: (a) pulsed arterial saturation, (b) PCEF, (c) subjective respiratory discomfort (RD) using a Borg scale (0=absolute well-being, no symptoms, 10=maximum sensation of discomfort).23 The subjects were asked to record on a diary card, each morning, any change in respiratory and clinical condition and evidence of RDE defined as: acute respiratory derangement with unresolved desaturation<95% despite patients independently trying to revert desaturations by increasing NIV16 (when prescribed) and assisted coughing techniques, fever with intercurrent respiratory tract infection, need to increase time of NIV >16h/day, severe secretion encumbrance with antibiotic prescription, significant increase (>5sessions/day) of manual assisted coughing techniques with air-stacking, urgent call out of family doctor, access to Emergency Room with or without need for hospitalisation.
At the end of each patient's follow-up period, the following data were collected: days of follow-up, number of RDEs, monthly decline in PCEF. In subjects presenting RDEs, values of PCEF, SpO2 and RD at three fixed time points −16 days before a RDE, the day before a RDE and 30 days after RDE starting or after 30 days since ER or hospitalisation event were concurrently evaluated.
Good adherence to the protocol was defined when patients performed at least 50% of prescribed daily protocol measurements.
Statistical analysisData were evaluated by statistical software STATA 11.2. PCEF decline was calculated as the difference between the last available PCEF value and the pre-discharge PCEF value; monthly decline was calculated as the whole decline/number of months of follow-up for each patient. A patient with high/low PCEF monthly decline was defined as a patient with a value higher or lower than the median value of monthly decline. A χ-square Pearson test was used to compare the groups with or without RDEs in subjects with high or low decline, and PCEF > or <270L/min. One-way ANOVA test was conducted among values of PCEF, SpO2 and Borg measured 16 days before, the day before a RDE and 30 days after a RDE and, post hoc analysis by Tukey Test was performed, if Fisher test was significant. Comparison of baseline continuous variables was conducted by Wilcoxon test in subjects with and without RDE. Compliance with the daily protocol measurements was defined as the ratio of the number of performed measurements divided by the total prescribed measurements (maximum #=270).
ResultsFrom April 2009 to July 2012, 18 subjects with ALS who met eligibility criteria were identified. Four subjects refused to participate and two subjects withdrew consent. Therefore, data for 12 subjects were analysed (66.7% of acceptance). Anthropometric, functional and ABG data at baseline are shown in Table 1. During an average period of follow-up of 125±102 days (range 21–270), the 12 subjects performed a total of 1175 measures. The total compliance for all abovementioned parameters was 37±32% (range 8–100%) worsening across time from 63±27% (at months 1–3) to 26±39% (at months 3–6), and (22±35%) at months 6–9. During the study, the subjects sent data reported on the diary mainly by e-mail (69%).
Anthropometric and functional characteristics of 12 patients with ALS (and caregivers) at hospital discharge.
| Variables | Values |
| Age, years (mean±SD) | 53±10 |
| Male/female, % | 75/25 |
| Years of disease, n (mean±SD) | 3.4±1.9 |
| ALS FRS-R (mean±SD) | 20.2±6.8 |
| NIV users, n | 7 |
| FEV1, % predicted (mean±SD) | 75±29 |
| FVC, % predicted (mean±SD) | 74±30 |
| FEV1/FVC (mean±SD) | 85±11 |
| VC, % predicted (mean±SD) | 75±31 |
| MIP, % predicted (mean±SD) | 34±17 |
| MEP, % predicted (mean±SD) | 34±13 |
| PaO2, mmHg (mean±SD) | 82±13 |
| PaCO2, mmHg (mean±SD) | 42±9 |
| pH (mean±SD) | 7.42±0.05 |
| SpO2, % (mean±SD) | 95.50±1.58 |
| PCEF, L/min (mean±SD) | 290±72 |
| Respiratory discomfort (Borg scale, mean±SD) | 2.92±1.31 |
| Caregivers age, y (mean±SD) | 49±11 |
| Time spent by caregivers, h/day (mean±SD) | 18.6±5.6 |
| Type of caregiver, % | |
| Wife/husband | 66 |
| Brother/sister | 5 |
| Son/daughter | 16 |
| Professional caregiver | 5 |
| Other | 8 |
ALS FRS-R: amyotrophic lateral sclerosis functional rating scale revised; FEV1: forced expiratory volume at first second; FRS: functional rating scale; FVC: forced vital capacity; MEP: maximal expiratory pressure; MIP: maximal inspiratory pressure; PaO2: pressure partial pressure of oxygen in arterial blood; PaCO2: pressure partial pressure of carbon dioxide in arterial blood; PCEF: peak cough expiratory flow; SpO2: oxygen saturation; VC: vital capacity.
16% of subjects delivered data by personal contact, and the remaining provided over telephone. 58% of subjects, because of delay in delivery, had to be followed up by phone at least once by PT and they performed PCEF with caregiver assistance.
One patient (8%) presented clinical instability not related to respiratory causes. Five out of the 12 subjects studied (42%) reported six episodes of RDEs: one episode of chest infection with fever needing antibiotics at home, three episodes of desaturation and disturbed sleep with urgent need to also increase time of NIV during the day without any change in NIV setting, one episode of severe secretion encumbrance with activation of more frequent use of manual assisted cough manoeuvres without any change in device setting (repetitive two hours application for 36 consecutive hours), one episode of respiratory failure needing hospitalisation and need for 24h NIV use. Two consecutive episodes, both due to urgent need to increase time of NIV, were reported by the same patient after 21 and 92 days from the start of the study. The mean time before the first RDE was 136±108 days (range 21–255 days). None of the subjects received mechanical cough assistance devices or needed tracheostomy during RDEs. Hospitalisation rate was 8.3% while none was admitted to ER and immediately discharged.
Table 2 shows differences in pre-discharge baseline data according to subjects with or without RDEs: among all these data, subjects with RDEs showed a statistically worse FEV1 and more years of disease duration.
Differences in baseline data according to patients with or without respiratory deterioration events (RDEs).
| Parameters | RDEs group (n=5) | No RDEs group (n=7) | p |
| Age, years | 51±5 | 54±12 | |
| Male, % | 71 | 80 | NS |
| FEV1, % predicted | 59.00±27.76 | 91.20±21.99 | 0.0472 |
| FVC, % predicted | 56.00±28.13 | 88.83±22.85 | NS |
| FEV1/FVC | 89.75±11.73 | 79.75±8.01 | NS |
| VC, % predicted | 59.40±31.03 | 86.71±27.93 | NS |
| MIP, % predicted (mean±SD) | 29.60±16.11 | 37.14±18.11 | NS |
| MIP, cmH2O | 31±14.71 | 40.5±24.75 | NS |
| MEP, % predicted (mean±SD) | 33.20±10.21 | 32.57±14.75 | NS |
| MEP, cmH2O | 61.25±15.10 | 61.25±17.69 | NS |
| PCEF, L/min | 276±80 | 286±43 | NS |
| PaO2, mmHg | 78.40±18.19 | 84.66±3.49 | NS |
| PaCO2, mmHg | 42.00±13.09 | 41.74±3.68 | NS |
| pH | 7.43±0.08 | 7.42±0.01 | NS |
| Years of diseases, years | 4.60±1.52 | 2.58±1.72 | 0.0384 |
| PCEF month/decline, L/min | 26.45±33.88 | 6.95±31.28 | NS |
| Follow-up, days | 150.4±92.95 | 107.42±111.6 | NS |
| ALS FRS-R, score | 18.4±0.87 | 19.28±3.42 | NS |
ALS FRS-R: amyotrophic lateral sclerosis functional rating scale revised; FEV1: forced expiratory volume at first second; FRS: functional rating scale; FVC: forced vital capacity; MEP: maximal expiratory pressure; MIP: maximal inspiratory pressure; NIV: non invasive ventilation; PaO2: pressure partial pressure of oxygen in arterial blood; PaCO2: pressure partial pressure of carbon dioxide in arterial blood; PCEF: peak cough expiratory flow.
Data are expressed as mean±SD.
The overall decline in PCEF during the study was −46.25±68.37L/min (range 30–190). Level of monthly decline in PCEF was −15.08±32.43 (median value 7.53; range 30–85.5)L/min. In our series, the lower level of PCEF was 40L/min and the minimum SpO2 was 90%. No differences were found in subjects with or without RDEs between subjects with high or low decline (p<0.079), PCEF > or <270L/min (p<0.276).
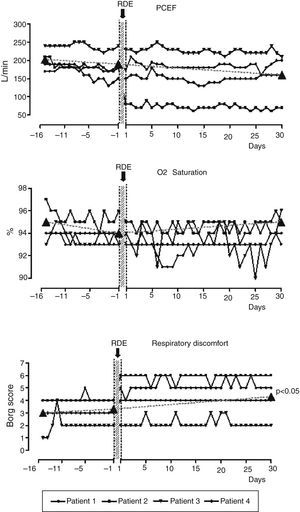
Subjects with high and low PCEF decline did not differ at the start of the project for any of the baseline variables. Individual trends and values in PCEF, SpO2 and RD at specific time points (16 days before, at the RDEs start and 30 days after RDEs) were available in 4 out of 5 subjects presenting an RDE (80%) (Fig. 1). ANOVA analysis shows that the sensation of discomfort only worsened significantly from 16 days before and 30 days after from 3.0±1.41 to 4.25±1.71 points of Borg score (Tukey Test significant). On the day of the RDE, these subjects had worsened PCEF (from 225±69.5L/min to 177.5±42.7L/min), SpO2 (from 95±1.83 to 93±0%) and subjective RD (from 2.25±0.96 to 4.75±1.89 Borg score) with respect to their pre-discharge data. These changes however did not reach statistical significance.
Individual trends and values in PCEF, SpO2 and respiratory discomfort by Borg scale 16 days before, the day before and 30 days after four RDEs related to respiratory causes in 4 representative subjects. Borg scale detected wellbeing respiratory sensation (0=absolute wellbeing, no symptoms, 10=maximum perceived feeling of discomfort).
PCEF indicates peak cough expiratory flow; SpO2, oxygen saturation as measured by pulse oxymetry.
Previous studies have shown that PCEF is a good functional indicator of spontaneous cough effectiveness.6–8
Home patients’ diaries have been proposed for COPD exacerbation24–27 while home variation in peak expiratory flow has been evaluated in asthma28,29 and COPD.30
Sancho et al.6 reported that ALS subjects received a home protocol based on scheduled clinical and functional assessment by a physician and were encouraged to request hospitalisation if they suffered dyspnoea, ineffective cough or decreased oxyhemoglobin saturation.6
A significant decrease of sniff nasal inspiratory pressure (SNIP) and a parallel increase in dyspnoea Borg Score were observed in 14 ALS subjects during a period of 19 months.31
Baseline dyspnoea index was found to be related with a decline in forced vital capacity which was better than that of the revised ALS functional rating scale (ALS-FRS-R) and a visual analogue scale.2
Compliance with the proposed protocol was not high; it was 67% before discharge and it got worse over time; this result is very similar to the compliance with home PCEF monitoring in subjects with asthma.32 Two main reasons can be identified: the long duration of the study and the huge number of measurements requested daily. Psychological and depressive reasons might have further reduced adherence.
In regards to the feasibility of conducting the protocol at home, it is interesting to note that the adherence to the protocol deteriorated, especially after the first three months, and that telephone feedback by the physiotherapist became necessary. Moreover, subjects sent their data through the mail thinking that this method was simpler and more reliable. In more than half of the cases caregivers performed the required measurements: this is not surprising since ALS is a severe disabling disease requiring a high amount of caring by caregivers.
More than 40% of subjects presented 6 episodes of respiratory derangements. Five subjects presenting a RDE were, in general, more compromised in all functional and clinical parameters. Also the decline during follow-up was worse than that of subjects without RDE.
Due to the small sample size, statistical significance was only found for FEV1 and years of diseases. It is also interesting to note that a cut off of baseline PCEF > or < to 270L/min was not predictable for RDEs.
Previous results from literature do not show what could happen to these subjects during a respiratory tract infection.18 Only Poponick et al.3 evaluated respiratory function in subjects with multiple muscular dystrophies during and in the post recovery phase of acute upper respiratory tract infection: whereas we monitored respiratory function, SpO2 and cough ability before, during and for a longer post recovery phase in a more homogeneous ALS population.
As demonstrated in 4 representative subjects (Fig. 1) only the sensation of discomfort worsened significantly from 16 days before and 30 days after the RDEs demonstrating that objective data present a faster recovery time than subjective ones.
Our pilot data do not support the hypothesis that subjects with ALS with low or high decline in PCEF during time and high or low level of baseline PCEF are prone to have a RDE.
The strengths of this study are: (a) proposal for a home daily cough capacity follow-up, (b) information on monthly trends in PCEF decline, (c) documentation of events during a RDE and during recovery time.
An important limitation to this study is the small sample size of the population: as a descriptive case-series we need to temper our interpretation of presented data. Poor protocol adherence prevents definitive conclusions as we need to consider that non-adherent patients could be the patients with the worst disease decline. We cannot exclude the possibility that a simpler and less cumbersome protocol (monitoring variables less frequently, monitoring fewer variables and simplifying the reporting) would improve patient compliance.
In conclusion, in subjects with ALS, good adherence to combined daily self-monitoring for PCEF, SpO2 and respiratory discomfort seems difficult to obtain and does not add further advantages to current practices of advising on clinical derangements; that is use of mechanical cough assistance or increase of ventilatory support immediately SpO2 drops below 95%. Confirmatory larger studies are necessary in this field in order to study new domiciliary monitoring tools and to find predicted clinical variables of respiratory distress events.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The Authors thanks Laura Comini for editing and critical revision and Alessandro Bettini for the English revision of the manuscript.
The study was performed at Fondazione Salvatore Maugeri IRCCS Lumezzane (BS) Italy.