Sarcoidosis is a rare granulomatous disease of unknown aetiology belonging to the wide group of interstitial lung diseases.). Although the limitlessness of BAL fluid is debated, it remains one of the best matrices for studying the pathogenesis of sarcoidosis. Natural killer (NK) cells have been described in BAL fluid from sarcoidosis patients. Elevated NK cells in BAL fluid from sarcoidosis patients have been found to be associated with poor outcomes. In this study, NK cells were evaluated in BAL samples from sarcoidosis patients at the time of diagnosis and associated with clinical characteristics in order to evaluate their prognostic role. Of the 276 patients suspected to have sarcoidosis on the basis of clinical and radiological findings, 248 had a final diagnosis of sarcoidosis. Clinical parameters, Scadding stage, and extrapulmonary localization were collected in a database. It resulted in fibrotic sarcoidosis patients being associated with an increase in lymphocyte percentages in BAL samples, particularly NK cells when compared with other groups. From ROC analysis, NK cell percentages in BAL samples resulted as being the best predictive markers in discriminating stage 4 of sarcoidosis from other RX stages (AUC=0.85, p<0.0001). Furthermore, after the stratification of patients on the basis of the number of extrapulmonary localizations, patients with an higher number of extrapulmonary localizations also showed higher percentages of NK cells in BAL fluid. In conclusion, NK cell percentages in BAL fluid can be considered a good prognostic marker of fibrotic phenotypes of sarcoidosis and involvement of other organs, although their diagnostic utility was poor.
Sarcoidosis is a rare granulomatous disease of unknown etiology belonging to the wide group of interstitial lung diseases (ILDs). Its clinical manifestations may range from asymptomatic to multiorgan impairment.1–3 Sarcoidosis features non-caseating epithelioid cell granulomas. Diagnosis is based on a compatible clinical presentation, non-necrotizing granulomatous inflammation in one or more tissue samples, and exclusion of other causes of granulomatous disease.4,5 Although efforts have been made to improve diagnosis, prognosis, and treatment, the lack of biomarkers remains a challenge for research.
A typical feature of pulmonary sarcoidosis is an accumulation of T‐helper cells in the lung, manifesting as lymphocytosis and an elevated lymphocyte CD4/CD8 ratio in bronchoalveolar lavage (BAL) fluid.6,7 Although the limitlessness of BAL fluid is debatable, it remains one of the best matrices for studying the pathogenesis of sarcoidosis, as demonstrated by recent publications.8,9 The cell component of BAL fluid includes alveolar macrophages, lymphocytes, neutrophils and eosinophils.10 Besides increased lymphocyte percentages, an altered CD4/CD8 ratio is a useful finding in the diagnosis of sarcoidosis, although it has limited accuracy.11
Moreover, other novel markers were proposed recently, of these, Natural killer (NK) cells have been described in BAL fluid from sarcoidosis patients resulting in decreased levels when compared with other ILD with fibrotic progression.12,13 In fact, another study demonstrated elevated percentages of NK cells in BAL from sarcoidosis patients which have been found to be associated with a poor outcome and a higher probability of requiring corticosteroid treatment.14
Frye BC and colleagues recently reported the use of a new score called Bronchoalveolar Cytology Threshold (BCT) to distinguish healthy persons from those with lung diseases.15 The index considers all four cell populations present in BAL samples. In a previous study, we investigated the diagnostic potential of BCT for distinguishing different interstitial lung diseases. Although we found limited accuracy for differentiating ILDs,16 samples from sarcoidosis patients showed a particular pattern, namely high heterogeneity with respect to other ILDs. Here we have improved the description of BCT score and NK cells observed in BAL samples from sarcoidosis patients at the time of diagnosis. These markers were for the first time evaluated in relation to subsets of sarcoidosis including Scadding stages and the relationship with extrapulmonary localizations.
Materials and methodsStudy populationsThis prospective monocentric study was conducted at the Siena translation research laboratory. The patients were in the care of Siena Regional Referral Centre for Sarcoidosis and other Interstitial Lung Diseases. A total of 1467 BAL samples were screened between January 2018 and June 2023. The samples were obtained by bronchoscopy for diagnostic purposes from patients suspected of havinginterstitial lung diseases.
The sarcoidosis diagnostic work-up and diagnosis of patients were performed in a context of multidisciplinary discussion, considering a combination of clinical, histological, immunological, radiological and lung function data.17 In the case of patients for whom histological sampling was not available or possible, a diagnosis of sarcoidosis was reached by multidisciplinary evaluation of clinical and radiological features in order to exclude other possible diseases, as recently endorsed by American Thoracic Society guidelines.18,19 Scadding radiological stages were defined as follows: stage 0, normal; stage 1, bilateral hilar adenopathy without parenchymal involvement; stage 2, bilateral adenopathy and parenchymal infiltration; stage 3, parenchymal infiltration; stage 4, pulmonary fibrosis associated with sarcoidosis according to international criteria.8,20 Demographic data, including age, sex, smoking habits, functional parameters (FEV1, FVC, TLC and DLCO percentages), BAL cell count with lymphocyte immunophenotyping and extrapulmonary localizations were recorded in an electronic database.
The study complied with the Declaration of Helsinki. All patients gave their written informed consent to participate in the study, which was approved by our local ethics committee (CEAVSE, PAN_HUB_1_17431).
Bronchoscopy and BAL processingBronchoscopy with bronchoalveolar lavage was performed for diagnostic purposes in line with the ATS/ERS Guidelines on BAL.21 BAL fluid was obtained by the instillation of four 60-ml aliquots of the saline solution via fibro-bronchoscope (Olympus IT-10) wedged in a subsegmental bronchus of the middle lobe or lingula. The first sample was kept separate from the others and was not used for immunological tests. BAL processing was performed by an experienced technician as previously reported.22
Evaluation of BCTThe most representative cell types in BAL, including alveolar macrophages, lymphocytes, neutrophilic and eosinophilic granulocytes, were analysed together to determine the Bronchoalveolar Cytology Threshold (BCT), which is expressed by the following formula: BCT=max(85-AM;0) +max(lymph-12;0) +max(neutro-2;0) +max(eos-1,0) where AM stands for alveolar macrophages (%), lymph stands for lymphocytes (%), neutro for neutrophils (%) and eos for eosinophils (%), as previously proposed by Frye et al. and Bergantini et al.16
Statistical analysisFor comparison of groups, the Mann-Whitney U test was used when two groups were comparable, while the Kruskal-Wallis test with Dunn test were used for continuous variables. For categorical variables, Fisher's exact or chi-squared test were used. Binomial logistic regression models and ROC curve analysis were used to determine the best model and cells for selecting variables that identified fibrotic sarcoidosis and the cells best associated with extrapulmonary localizations. A p-value <0.05 was considered statistically significant. GraphPad 9.0 software and Jamovi 2.3.26-free software were used for the analysis.
ResultsStudy populationOf the 276 patients with suspected sarcoidosis on the basis of clinical and/or radiological findings, 248 had a final diagnosis of sarcoidosis. The other 28 patients with different diagnoses were used as controls. To establish the final diagnosis, histological sampling was available for 88 % of patients and Scadding stage was available for 198 patients (14 stage 0, 20 stage 1, 129 stage 2, 17 stage 3 and 18 stage 4 patients).
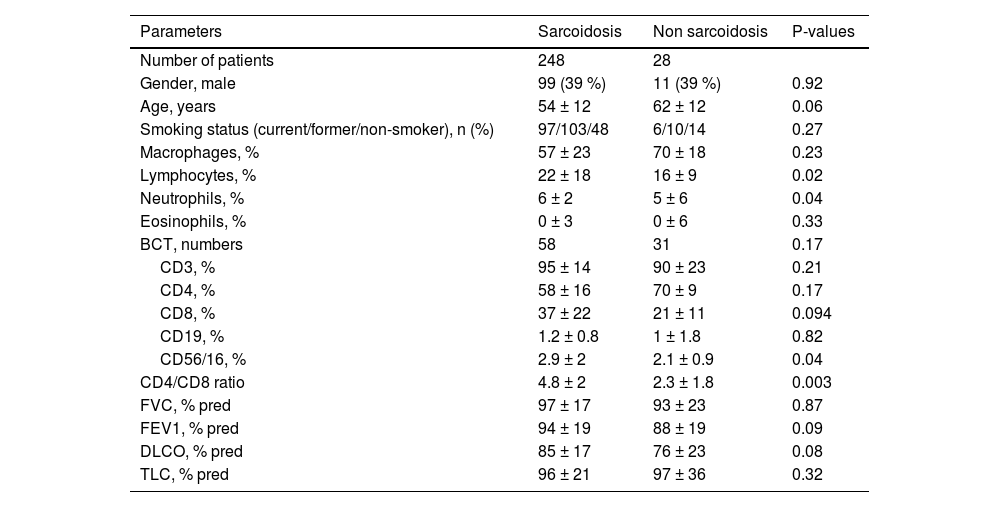
Lymphocyte percentages and CD4/CD8 ratio were significantly higher in the sarcoidosis group than in controls, while other parameters, including lung function (FEV1, DLCO, FVC and TLC), age, sex, and smoking did not differ between the two groups (Table 1).
Clinical and lung function parameters in the two groups. Results are expressed as mean± standard deviation (m ± S.D.).
Abbreviations: BCT: Bronchoalveolar Cytology Threshold, CD: cluster of differentiation, FVC: forced vital capacity, FEV1: Forced Expiratory Volume in the first second, DLCO: diffuse capacity of carbon monoxide, TLC: total lung capacity.
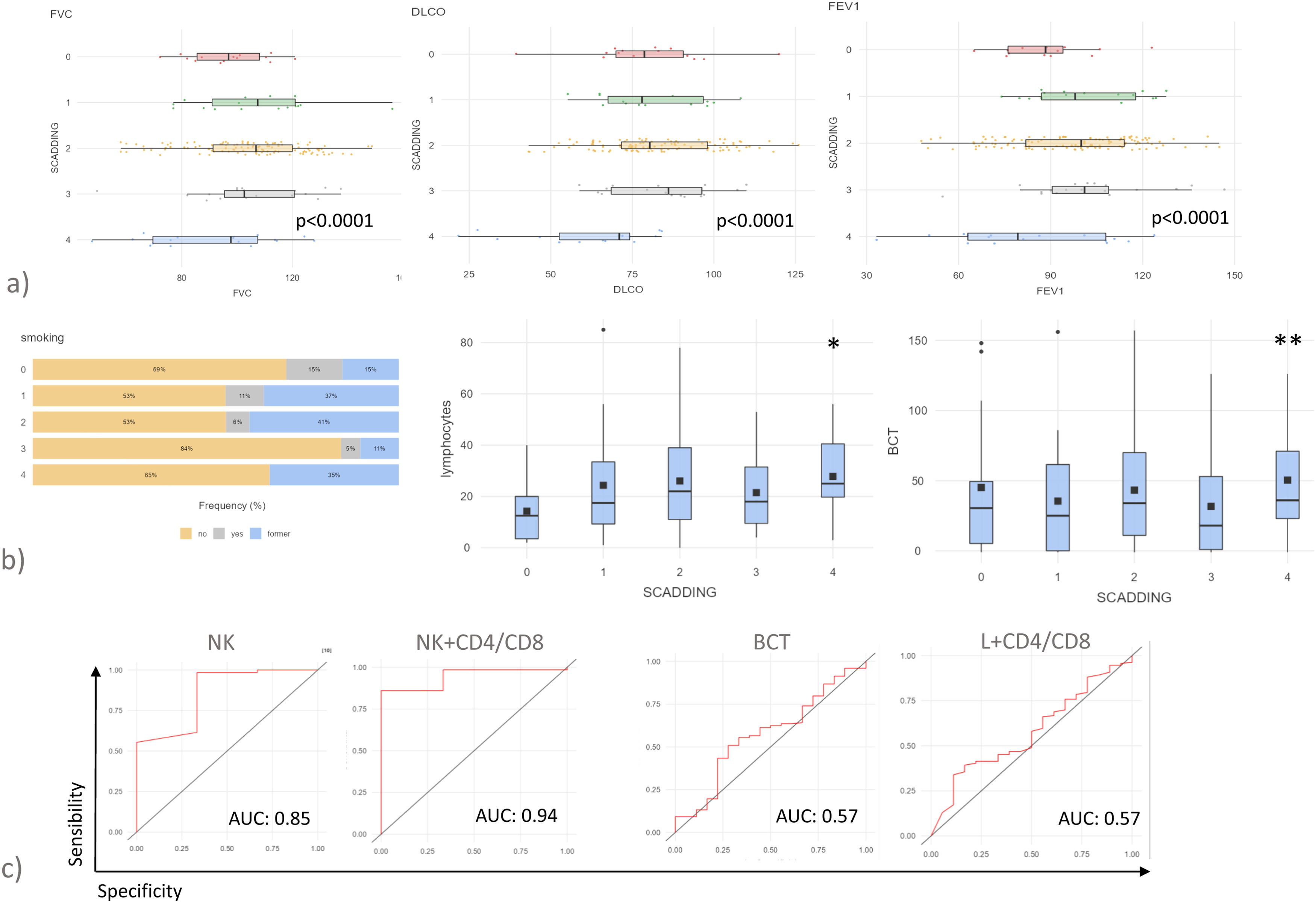
Patients with sarcoid lung fibrosis (Scadding stage 4) showed respiratory impairment, expressed by lower FEV1, FVC and DLco percentages than patients of the other group (Fig. 1a). In line the literature, stages 3 and 4 were associated with a prevalence of never-smokers (Fig. 1b). Stage 4 was associated with an increase in lymphocyte percentages in BAL samples, particularly NK cells and BCT was also elevated (Fig. 1b).
(a) Histograms showing pulmonary function tests parameters, FVC, FEV1 and DLCO of patients with sarcoidosis stratified by Scadding stage. P values represent the result of Kruskal-Wallis test (b) Frequency of smoking habits, lymphocytes percentages of BAL and BCT score of patients with sarcoidosis stratified by Scadding stage. P values represent the result of Kruskal-Wallis test (c) ROC curve analysis considering fibrotic sarcoidosis vs other RX stages.
*p<0.05, **p<0.01, ***p<0.001 and **** p<0.0001. Unless otherwise indicated, p values are not significant. The data in histograms reported individual values, mean (centre bar) ± SEM (upper and lower bars). Abbreviations: AUC: area under the curve, BCT: BAL cytology threshold, NK: natural killer.
Patients with fibrotic sarcoidosis were therefore placed in a separate group in order to find the biomarker most predictive of this complication. Only NK cells proved suitable for predicting fibrotic sarcoidosis (AUC=0.85, p<0.0001). NK cell percentages combined with CD4/CD8 ratio in a logistic regression model significantly increased the AUC (AUC=0.94, p<0.0001) (Fig. 1c). BCT and the logistic model of lymphocyte percentages and CD4/CD8 ratio failed to predict fibrotic sarcoidosis (Fig. 1c).
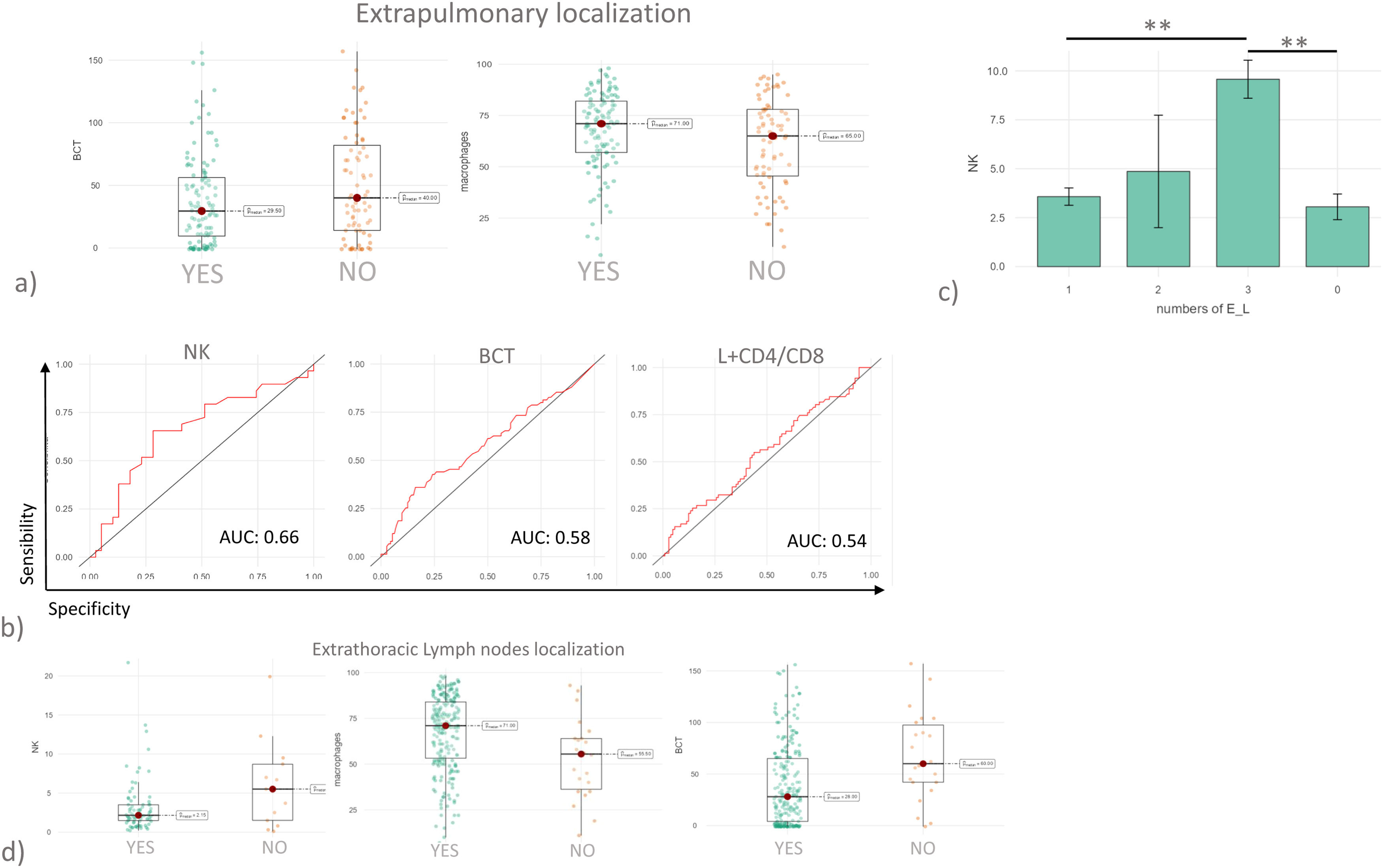
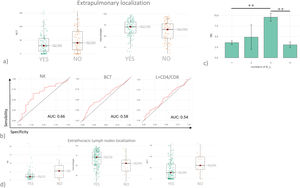
Stratification by extrapulmonary localizationData on extrapulmonary localizations (E_L) was available for 184 patients, 75 with E_L and 109 without. The organs involved in Sarcoidosis were reported in a supplementary Table 1. BCT and macrophages proved to be the only variables able to distinguish the two groups (Fig. 2a). As reported in Fig. 2b, the logistic model indicated that the combination of lymphocytes and CD4/CD8 ratio (AUC= 0.54) was not able to discriminate patients with E_L; nor was BCT (AUC=0.58). Also, in this case NK cells proved to be the marker most predictive of E_L, with a fair degree of accuracy (AUC=0.66) and good specificity (0.87). When patients were further stratified on the basis of number of E_L, patients with a higher number of E_L also showed higher percentages of NK cells in BAL fluid (Fig. 2c). Patients with extrathoracic lymph node localizations showed significantly higher percentages of NK cells and macrophages and significantly higher BCT scores (Fig. 2d).
(a) Histograms showing BCT and macrophages values in sarcoidosis cohort after stratification based on the presence of extrapulmonary localizations. (b) ROC curve analysis considering patients with extrapulmonary localization vs patients without. (c) Histograms reported NK cell percentages in BAL in patients with sarcoidosis stratified based on the number of extrapulmonary localizations. (d) Histograms reported NK, macrophages, and BCT values in BAL of patients with sarcoidosis stratified based on extrapulmonary involvement of lymph nodes.
*p<0.05, **p<0.01, ***p<0.001 and **** p<0.0001. Unless otherwise indicated, p values are not significant. The data in histograms reported individual values, mean (centre bar) ± SEM (upper and lower bars). Abbreviations: AUC: area under the curve, BCT: BAL cytology threshold, NK: natural killer, E_L: extrapulmonary localization.
Sampling the human respiratory tract by BAL is a means of obtaining cells from the airways and alveoli of patients and controls for research and clinical purposes. The utility of BAL in the diagnosis of ILD is still debated. In sarcoidosis, BAL parameters can be of diagnostic utility, especially lymphocytosis and elevated CD4/CD8 ratio. Recent literature reports novel biomarkers find in BAL cell count or supernatant able to support diagnosis, prognosis, and prediction to treatments.23 Among these, the percentage of NK cells showed a promising association with sarcoidosis.1 In fact, in a previous paper, we demonstrated a lower percentage of NK cells in patients with sarcoidosis than in those with progressive fibrotic ILDs, such as IPF, cHP and CTD-ILD.24 In the present study, when patients were stratified by Scadding stage, we observed that fibrotic sarcoidosis (Scadding stage 4) was associated with higher percentages of NK cells than the other stages, suggesting that these cells are linked to the development of fibrosis. NK cells proved to be associated with poor outcomes and advanced radiological stage.14 In a small cohort, it was recently reported that NK cell percentages in BAL fluid increased with the severity of sarcoidosis.25 Sarcoidosis patients requiring steroid treatment therefore also had higher percentages of NK cells in BAL fluid.14 Overall, these reports agree on a potential role of NK cells in the development of a more aggressive disease phenotype. It has been postulated that release of IFN-γ by NK cells may stimulate TNF-α secretion by macrophages, further increasing the Th1 response and perpetuating sarcoid inflammation.26
In the present study, the association with CD4/CD8 also showed good performance in discriminating fibrotic sarcoidosis. The diagnostic utility of the CD4/CD8 ratio is widely accepted.27 Sarcoidosis patients are reported to have an elevated CD4/CD8 ratio with 54–80 % sensitivity and 59–80 % specificity.11 Although the CD4/CD8 ratio is often used in the diagnosis of sarcoidosis, it may not reflect disease severity,6 whereas in combination with NK cell percentages a very good model was produced for predicting the most severe forms of sarcoidosis.
Regarding extrapulmonary localizations, BCT and macrophage percentages were significantly different in patients with and without such localizations. Multisystemic sarcoid involvement (expressed as number of extrapulmonary localizations) appeared to be correlated with an elevated percentage of NK cells in BAL fluid. It was recently reported that peripheral blood NK cells in sarcoidosis are associated with early cardiac involvement28 but little data is available on links between BAL parameters and extrapulmonary localizations.
In conclusion, we found that NK cell percentages in BAL fluid were a good prognostic marker of fibrotic phenotypes of sarcoidosis and involvement of other organs, although their diagnostic utility was poor. More detailed study of NK cell subsets in BAL fluid may provide useful information on the pathogenetic involvement of these cells in granuloma formation and allow better management of patients requiring corticosteroid therapy.
CRediT authorship contribution statementL. Bergantini: Conceptualization, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. M. Spalletti: Methodology, Writing – original draft, Writing – review & editing. M. d'Alessandro: Conceptualization, Investigation, Writing – review & editing. M. Genovese: Methodology, Writing – review & editing. E. Masotto: Methodology, Writing – review & editing. P. Cameli: Investigation, Writing – review & editing. A. Prasse: Writing – original draft, Writing – review & editing. E. Bargagli: Project administration, Writing – original draft, Writing – review & editing.