Malignant pleural effusions are an important burden of malignant disease. Slurry talc pleurodesis remains one of the most common and effective therapeutic options.
AimInvestigate the predictive factors related with the efficacy of this technique in malignant pleural effusions.
MethodsRetrospective analysis of all pleurodesis performed during a 10-year period in a Pulmonology Unit. All demographic and clinical data were collected, including the histologic tumoral type and the biochemical, microbiological and cytological fluid features. Efficacy was defined as the lack of recurrence of pleural effusion. It was used Kaplan–Meyer analysis to estimate overall survival.
ResultsFrom a total of 202 patients submitted to pleurodesis (47% men; mean age 66.9±12.02 years). Light's criteria identified 86.6% as exudates. We found 85.1% survival at 30-day post-pleurodesis, which means the therapy used has significant success. A logistic regression model applied explained that variance in post-pleurodesis events was mostly due to age and gender rather than pleural biochemical factors (X2(5)=44.648, p<0.001, R2 28.3%).
ConclusionThis study suggests that clinical evaluation of biochemical values, bacteriological results and malignant tumor diagnosis may not be enough to predict post-pleurodesis relapse with high accuracy. Furthermore, we observed, in ten years of pleurodesis performed in our Hospital, that pleurodesis is an effective life prolonging therapy for patients that fit the criteria for this intervention.
Malignant pleural effusions (MPE) are an important burden of malignant disease, leading to a significant reduction of quality of life with progressive dyspnea, dry cough, chest pain and reduced physical activity.1–4 Lung and breast neoplasms are responsible for 75% of all MPE and the mean survival time is 3–13 months.5–9
Despite management of underlying malignancy with chemo/radiotherapy, MPE may persist or recur and requires palliative interventions in order to control or alleviate the symptoms.2,6,10
Several palliative treatment options are available including thoracentesis or talc pleurodesis.2,6,10 Thoracentesis is easy to perform, but has a 98% recurrence rate at 30 days.6,11 Pleurodesis prevent re-accumulation of the effusion and thereby of symptoms, and avoid the high cost and physical and emotional trauma caused by repeated hospitalization of thoracocentesis.1,12 Several techniques and various agents have been used for this purpose, with different efficacy. Commonly used sclerosants are talc, tetracycline derivatives and bleomycin. Talc pleurodesis gives a success rate of 81 to 100%, which is in contrast to 65 to 76% achieved with tetracycline and its derivatives and 61% for bleomycin.13,14 Talc pleurodesis can be made by talc slurry via tube thoracostomy or talc insufflation via thoracoscopy.15–20
Unfortunately, pleurodesis fails in 10–40% of patients, with associated marked cost and morbidity. So, the identification of various clinical and biochemical parameters in predicting pleurodesis outcomes, may help to identify which patients would benefit the most from pleurodesis.21,22
The purpose of this study is to investigate the predictive factors related to the efficacy of slurry talc pleurodesis in malignant pleural effusions.
MethodsThe researchers did a retrospective analysis of all talc pleurodesis performed during a 10-year period in a Pulmonology Unit at Centro Hospitalar e Universitário de Coimbra.
Talc slurry administration via chest tubeIn all cases, pleurodesis was performed by talc slurry via a chest tube. A dose of 5 grams of sterile, asbestos-free talc (Steritalc® F2, manufactured by Novatech, France) mixed with 90ml of sterile saline and 10ml of lidocaine 1% was instilled through the chest drain, which was clamped for 6h after the procedure. Chest drain was removed when the chest radiograph confirmed satisfactory lung expansion and the total 24-hour drainage was less than 150ml, with no air leak. Another chest radiograph was done for all patients a few hours post chest drain removal and if satisfactory, patients were discharged.
Data collectionAll demographic and clinical data available were collected, including the histologic tumor type and the biochemical (pH, LDH, albumin, proteins, glucose), microbiological and cytological fluid features. Efficacy was defined as the lack of recurrence of pleural effusion (relapse) during the period of analysis.
Statistical analysisWe used a Kaplan–Meyer analysis to estimate average survival. To identify factors affecting efficacy and survival we used univariate and multivariate analysis. Data were statistically described in terms of mean±standard deviation (±SD), or frequencies (number of cases) and percentages when appropriate. Comparison of numerical variables between the study groups was done using Mann Whitney U test for independent samples when data were not normally distributed. For comparing categorical data, Chi square (χ2) test was performed (p<0.05). All statistical calculations were done using computer program SPSS version 21 (95% Confidence Interval always assumed).
ResultsA total of 202 patients were submitted to talc pleurodesis (47% men; mean age 66.9±12.02 years). Light's criteria identified 86.6% as exudates and all patients had a positive cytology and histology for malignant disease (64.4% lung cancer; 15.3% breast cancer; 8.4% hematological cancer; 7.4% gynecological cancer; 2.5% intestinal cancer; 2% skin cancer). There was a distinct distribution of cases, where Lung Tumor was the most frequent among relapse and non-relapse post pleurodesis intervention, when compared to other histological tumor types. With the exception of skin tumor (with only 4 cases), we observed that post-pleurodesis non-relapse was more frequent among all groups (X2(5)=13,773; p=0.017). Pleurodesis was effective in 70.3% of all cases.
Bivariate analysisTwo types of analysis were performed: one considering cut-off values and their association with post pleurodesis results; and the other comparing mean values between post pleurodesis results.
There was a higher frequency of male patients with relapse than female patients (54.2%, n=59), but there was no statistically significant difference between groups, when comparing relapse events in relation to gender (X2(1)=1.506; p=0.278). Male patients were also 31.6% more likely to suffer from post-pleurodesis pleural effusion relapse (OR=0.684 [0.372;1.257]).
We evaluated correlation between different relevant variables for both post-pleurodesis events in order to identify influence factors on survival. Statistically, significant observations were found for the relapse group survival in relation to cell count (r=0.301, p=0.021). The non-relapse group survival had a weak correlation with proteins and LDH levels found at the pleural effusion (respectively, r=0.289 and r=−0.177), p<0.05.
Considering cut-off valuesWe applied Chi-square tests considering the cutoff values for biochemical results of the pleural fluid (pH, glucose, proteins, LDH, cell count). We observed a statistically significant association between post-pleurodesis relapse and all other variables, except LDH (Table 1).
Bivariate Analysis of categorical variables, comparing with post-pleurodesis relapse event.
| Relapse | ||||
|---|---|---|---|---|
| Yes (n=59) | No (n=143) | |||
| pH>7.20 | 59 | 127 | X2(1)=7.169* p=0.007 | |
| pH<7.20 | 0 | 16 | ||
| Glucose>60 | 56 | 117 | X2(1)=5.827* p=0.025 | OR=4.148 [1.204;14.289] |
| Glucose<60 | 3 | 26 | ||
| Proteins>3.1 | 59 | 118 | X2(1)=11.772* p=0.001 | |
| Proteins<3 | 0 | 25 | ||
| LDH<1000 | 55 | 122 | X2(1)=2.407 p=0.090 | OR=2.367 [0.776;7.222] |
| LDH>1000 | 4 | 21 | ||
| Cell count>1000 | 41 | 68 | X2(1)=8.092* p=0.005 | OR=2.512 [1.319;4.784] |
| Cell Count<1000 | 18 | 75 | ||
We applied non-parametric tests (due to K-S, p<0.05) to test the association between relapse and age and the biochemical results of pleural fluid (pH, glucose, proteins, albumin LDH, cell count). We observed a statistically significant association between post-pleurodesis relapse event and median cell count value (Table 2).
Bivariate Analysis of continuous variables, comparing with post-pleurodesis relapse event.
| Relapse | Man U | p value | ||
|---|---|---|---|---|
| Yes (n=59) | No (n=143) | |||
| Age | U=3669.500 | 0.146 | ||
| Median | 66 | 69 | Z=0.146 | |
| pH | U=3617.500 | 0.111 | ||
| Median | 7.56 | 7.52 | Z=−1.592 | |
| Glucose | U=3876.500 | 0.365 | ||
| Median | 93 | 98 | Z=−906 | |
| Proteins | U=3886.500 | 0.379 | ||
| Median | 4.2 | 4.5 | Z=−0.880 | |
| Albumin | U=4201.500 | 0.964 | ||
| Median | 2.5 | 2.7 | Z=−0.045 | |
| LDH | U=3731.000 | 0.197 | ||
| Median | 359.0 | 286.0 | Z=−1.291 | |
| Cell count | U=3230.000* | 0.009* | ||
| Median | 1300 | 1000 | Z=−2.619 | |
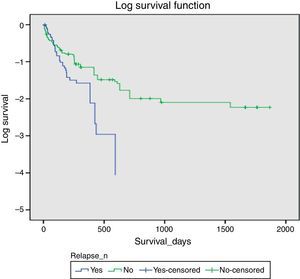
There was a significant difference in survival times between the groups (log rank test p=0.01, alpha<0.05). The Kaplan–Meier survival curve presents an average survival estimate for the non-relapse group of 400 days [295; 506 (IC95%)] and for the relapse group estimate was 170 days [127; 273 (CI95%)], with a 85.1% success rate at 30 days (n=47) (Fig. 1).
Explanatory factors analysisA logistic regression was performed to ascertain the effects of biochemical indicators in the pleural fluid (according to cut-off values), age, gender, type of diagnosis and fluid characteristics by cell count (transudate versus exsudate) on the likelihood that patients have post pleurodesis relapse (Table 3).
Results of binary logistic regression (95%CI), Hosmer Lemeshow (X2(8)=9.389, p=0.311).
| Variables | B-coefficient | Standard Error | Odds ratio | 95% CI | Wald (df 1) | p |
|---|---|---|---|---|---|---|
| Gender (categorical) | 0.909 | 0.345 | 2.481 | (1.261;4.881) | 6.922 | 0.009 |
| Age | 0.036 | 0.015 | 1.037 | (1.008;1.067) | 6.150 | 0.013 |
| Glucose pleural effusion (categorical) | −1.412 | 0.680 | 0.244 | (0.064;0.923) | 4.319 | 0.038 |
The logistic regression model was statistically significant (X2(5)=44,648, p<0.001). The model applied explained 28.3% (Nagelberke R2) of the variance in post-pleurodesis events and correctly classified 73.3% of cases. Males were 2.48 more likely to suffer relapse than females. Increasing age was associated with an increased likelihood of having a relapse event, but glucose below 60mg was associated with a reduction in the likelihood of exhibiting relapse events. [AUROC value=0.75 (95%C.I. 0.685 to 0.818)].
DiscussionIn a general hospital, twenty-five percent of all pleural effusions are secondary to cancer. Patients with cancers frequently develop recurrent MPEs secondary to their disease.20 Normally, more than 90% of MPEs are exudates.7 However, we found in our study 13.4% of transudates, which can be explained by the increase of hydrostatic pressure as a result of congestive heart failure, decrease in oncotic pressure from hypoalbuminia or increase in the normal negative pressure (more negative intrathoracic pressure) secondary to atelectasis.20
Talc pleurodesis is an effective treatment for the control of malignant pleural effusions (MPEs) to maintain the patient's quality of life, as many already suffer from poor general condition.5,13,15,20,23 Recent data have established that talc pleurodesis will fail in about 30% of patients,3 which is consistent with our results of 29.7% relapse post-pleurodesis.
In this study, we investigated the predictive factors related with the efficacy of slurry talc pleurodesis in MPEs.
Age and gender had no statistical significance between groups (p>0.05), but there was a tendency for males to have higher risk of suffering from pleural effusion relapse post pleurodesis interventions (31.6%).
Bivariate analysis of biochemical values reveals that some variables at pathological levels do not translate into higher relapse events. Alsayed et al.22 demonstrated that low pH and glucoses levels together with higher LDH level are related to poorer response to sclerosant agent and shorter mean survival. However, in a meta-analysis of the primary data from multiple cases series it was found that more than 50% of patients with low pleural fluid pH had successful pleurodesis. Therefore, pleural fluid pH had only modest predictive value for predicting symptomatic failure and should be used with caution.25,26
Glicosis and pH value had a significant statistical association with post-pleurodesis relapse events (p<0.05), but the pathological values of pH<7.30 or glucose<60mg/dl are more often found in the no-relapse group. This suggests that there might be other confounding factors that influence relapse events which were not taken into account like comorbidities such as diabetes. Pantazopoulos et al.24 in a retrospective analysis excluded diabetes mellitus and other causes of hyperglycemia and concluded that pleural glucose levels could be a reliable predictor of pleurodesis failure in patients without conditions that could lead to hyperglycemia.
When looking at biochemical values as continuous values and its difference when comparing post-pleurodesis groups, we observe that there is no statistically significant correlation. The exception was pleural fluid differential cell count, where a higher cell count was significantly associated with relapse group when compared to the non-relapse group (Med=1300 and Med=1000, respectively, p=0.009).
As expected, survival correlated with biochemical parameters, but not all factors were found to have significant correlation in each group, particularly in the relapse group. This might suggest that the relapse group had more confounding factors that might play an important role as predictors for relapse events which need to be taken into account when doing research on post-pleurodesis events. Indeed, survival had a negative correlation with glucose levels in the pleural effusion (r=−238, p=0.070) in the relapse group.
This sample had a median survival time consistent with the literature at 30 day post-pleurodesis survival (85, 1%).5,15
Considering that the mean survival time without treatment is 3–13 months,5–9 the pleurodesis technique applied was also found to have significant success for both groups – with or without relapse. The average survival period for the relapse group was 167 days [CI95%:(124.27;210.51) – P50=97 days] and for the non-relapse group average survival was 283.43 days [CI95%(215.71;351.14) – P50=141]. This means that optimal results were found after post-pleurodesis for the sample observed and pleurodesis remains a reasonable palliative option, as it provides a significant increase in survival chances (even with a relapse event) beyond the 3 months of survival without treatment.
A logistic regression model analysis was performed and showed age and gender as post-pleurodesis predictors of relapse events, while glucose cut-off variable was not a predictor for these events. One of the limitations of this study might be the sample size, in which small deviations can interfere with the model.
ConclusionThis study suggests that clinical evaluation of biochemical values, bacteriological results and malignant tumor diagnosis may not be enough to predict post-pleurodesis relapse with high accuracy. Even though age and gender might influence outcome, the application of the logistic regression model suggests that more factors are involved. In short, we observed in ten years of pleurodesis performed in our Hospital, a significant statistical increase in patient survival.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestAuthors declare no conflict of interest.