Alpha 1 Antitrypsin Deficiency (AATD) is a genetic disorder that results in reduced plasma levels and/or functionality of Alpha 1 Antitrypsin (AAT), a serine protease inhibitor. Deficiency in AAT predisposes patients to greater risk of early-onset chronic obstructive pulmonary disease/emphysema due to excessive proteolysis of lung parenchyma, in addition to liver disease as a result of polymerisation of certain mutant AAT proteins.1 Weekly intravenous (i.v.) infusion of human AAT (AAT therapy) is currently the only condition-specific treatment for AATD-associated disease, and clinical data suggest that AAT therapy may slow the progression of emphysema in patients with AATD.2
There is evidence indicating a role for AAT in conception and pregnancy. Indeed, AAT has been suggested as important to angiogenesis and vascularisation of the endometrium, as well as trophoblast invasion and embryo implantation.3 Associations between low AAT levels and pregnancy-related complications, such as preeclampsia, spontaneous abortion, and preterm labour, have also been described.4 Nevertheless, evidence is still limited regarding the clinical consequences of AATD on pregnancy, and less so regarding the use of AAT therapy in pregnant women. Here, we present a case detailing the clinical course of a pregnant woman with AATD, including the initiation of AAT therapy during pregnancy. The patient signed informed consent for use of her de-identified clinical data for research, analysis, and reporting.
The patient, a 31-year-old, underweight (BMI: 24.3), non-smoking female, had been diagnosed with AATD (Pi*SZ genotype) at the age of 21. She was tested for AATD following a lack of response to treatment for severe asthma, but had no family history of AATD. She had a complex clinical profile including a history of spontaneous pneumothorax, severe asthma and common variable immune deficiency. At the time of diagnosis, she refused AAT augmentation therapy due to practical difficulties related to infusion time and frequency of infusions. On average, she experienced ten moderate exacerbations a year requiring oral antibiotics and/or corticosteroids, and four severe exacerbations a year requiring hospitalization. The patient became pregnant at 29 years of age. Pulmonary Function Tests (PFTs) performed at her last pulmonology consultation prior to pregnancy (6 months pre-pregnancy) showed a severe restrictive ventilatory impairment. (Table 1). Serum AAT level was slightly reduced.
Time course of Alpha 1 Antitrypsin level and lung function. ERS Task Force Global Lung Initiative 2012 reference values [5] were used for lung volumes. (AAT= Alpha 1 Antitrypsin; FEV1= forced expiratory volume in the 1st second; FVC= forced vital capacity; NA= not available; TLC= total lung capacity).
| AAT level (mg/dL) | FEV1, L (% predicted) | FVC, L (% predicted) | TLC, L(% predicted) | |
|---|---|---|---|---|
| 6 months pre-pregnancy* | 74 | 0.79 (31) | 1.13 (31) | 2.77 (54%) |
| 9 weeks pregnant | NA | 1.90 (59) | 2.18 (59) | NA |
| 18 weeks pregnant⁎⁎ | 38 | 1.65 (51) | 2.06 (56) | NA |
| 23 weeks pregnant | NA | 1.46 (45) | 1.72 (46) | NA |
| 26 weeks pregnant | 108 | 1.7 (53) | 1.89 (51) | NA |
| 30 months post-partum | 68 | 1.90 (61) | 2.22 (62) | 4.87 (96) |
During pregnancy, pulmonology consultation, serum AAT testing and PFTs were performed on a regular basis. The patient experienced one moderate exacerbation at Week 17 of pregnancy, and at Week 18; moreover, a significant drop in her forced expiratory volume in the 1st second (FEV1) (- 0.25 L) compared to Week 9 value was recorded at Week 18. Serum AAT level was markedly reduced. So, intravenous (i.v.) augmentation therapy was initiated and was regularly continued throughout pregnancy at a dose regimen of 60 mg/kg/week aimed at maintaining serum AAT level consistently above the protective threshold of 80 mg/dL (11 μM/L).6 No adverse event associated with augmentation therapy was reported. The patient experienced a severe exacerbation at Week 28 of pregnancy; however, there were relevant improvements to respiratory function parameters (Table 1). Following consultation with a multidisciplinary care team on the risks (in particular severe respiratory tract infection) and benefits of continuing her pregnancy, the patient consented to undergo a caesarean section at 32 weeks, delivering a healthy baby.
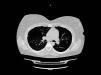
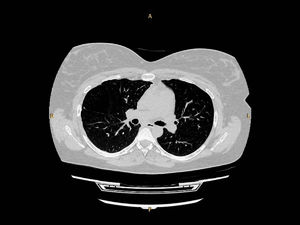
At a follow-up visit conducted 4 weeks post-partum, she reported mild exertional dyspnea. A mild exacerbation with uncomplicated clinical course was reported at 3-month follow-up. Over the next 2 years, exacerbations had reduced relative to pre-pregnancy/AAT therapy to three moderate and one severe per year on average. At the patient's latest consultation (30 months post-partum), PFT showed mild obstructive impairment with recovery of FEV1 to her pre-pregnancy values (Table 1). A lung CT scan was performed, showing a diffuse low attenuation area in the right upper lobe (Fig. 1).
Data regarding AATD and pregnancy are limited, possibly due to the underdiagnosis of women of childbearing age; indeed, patients with AATD are usually identified in their 40s or 50s.7 Complications associated with preterm labour, preeclampsia and spontaneous abortion and the risk of rapid decline in PFTs8 highlight a need for close monitoring of the patient throughout pregnancy.3 Care of pregnant women with AATD currently follows guidelines for general lung disease, with emphasis on the management of respiratory symptoms and prevention of exacerbations. Whether to initiate AAT therapy relies on expert opinion and clinical experience; however, there is currently little evidence to guide this decision.4 Although there are no known pregnancy-specific safety concerns with augmentation therapy, data for the use of AAT therapy in pregnancy are limited to a single recent case report by Gaeckle et al., which describes a patient who continued with AAT therapy throughout pregnancy and delivered a healthy baby at term.4
This case adds to the very limited data regarding AAT therapy during pregnancy, supporting the argument that it can be safely initiated in response to severe impairment of respiratory function. The case also adds to the evidence that it is possible for patients with AATD to experience no pregnancy-related complications and deliver a healthy baby; however, close patient monitoring is essential to a positive outcome.
Funding sourcesWriting support for this publication was funded by CSL Behring.
No study sponsor was involved in designing or conceptualising the study, in collecting, analysing/interpreting the data, in drafting the manuscript or in the decision to submit the manuscript for publication.
Author contributionsConceptualization: GG; data collection: AA and SL; data interpretation: AV; Writing – Review and editing: all authors.
Medical writing assistance was provided by Amy Adlard of Meridian HealthComms Ltd.