Invasive mechanical ventilation (IMV) represents a risk factor for the development of ventilator-associated pneumonia (VAP), which develops at least 48h after admission in patients ventilated through tracheostomy or endotracheal intubation. VAP is the most frequent intensive-care-unit (ICU)-acquired infection among patients receiving IMV. It contributes to an increase in hospital mortality, duration of MV and ICU and length of hospital stay. Therefore, it worsens the condition of the critical patient and increases the total cost of hospitalization. The introduction of preventive measures has become imperative, to ensure control and to reduce the incidence of VAP. Preventive measures focus on modifiable risk factors, mediated by non-pharmacological and pharmacological evidence based strategies recommended by guidelines. These measures are intended to reduce the risk associated with endotracheal intubation and to prevent microaspiration of pathogens to the lower airways.
A ventilação mecânica invasiva representa um fator de risco para o desenvolvimento da pneumonia associada ao ventilador (PAV), que se desenvolve 48 horas ou mais após a admissão hospitalar, em doentes ventilados através de traqueostomia ou intubação endotraqueal. A PAV é a infeção adquirida na unidade de cuidados intensivos (UCI) mais frequente entre os doentes submetidos a ventilação mecânica invasiva. Contribui para o aumento da mortalidade hospitalar, da duração da ventilação mecânica e do tempo de internamento na UCI e no hospital. Por conseguinte, agrava o estado de saúde do doente crítico e aumenta o custo total da hospitalização. A adoção de medidas preventivas é imprescindível, de modo a garantir o controlo e a diminuição da incidência da PAV. As medidas preventivas incidem sobre os fatores de risco modificáveis, sendo aplicadas estratégias não farmacológicas e farmacológicas baseadas na evidência e recomendadas por guidelines. As medidas preventivas têm como finalidade diminuir o risco associado à intubação endotraqueal e prevenir a microaspiração de microrganismos patogénicos para as vias aéreas inferiores.
Invasive mechanical ventilation is a risk factor for the development of pneumonia,1,2 being the ventilator-associated pneumonia (VAP) a public health problem. VAP is a hospital-acquired pneumonia which occurs in patients who were subjected to invasive mechanical ventilation, whether through tracheostomy or endotracheal intubation, at least 48 hours before the onset of infection and that were ventilated at the onset of the pneumonia.3 This disease is also classified according to the time elapsed from the beginning of the mechanical ventilation (MV) to the onset of pneumonia; it is considered as early-onset if it occurs within 4 days of the start of MV, and late-onset if it occurs after 5 or more days of MV onset.4,5 However, not all the studies consider early- and late-onset VAP within the same time range frame (Table 1).
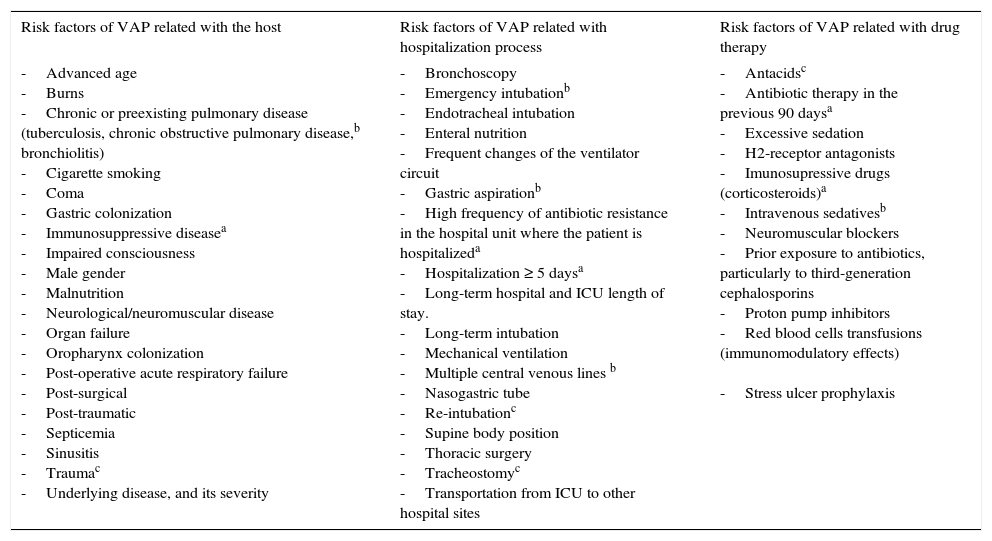
VAP occurs primarily in intensive care units (ICU),6 where the most debilitated patients are hospitalized, often requiring ventilatory support. It is estimated that from 8 to 28% of the patients receiving MV develop pneumonia, the risk is between 3 and 10 times higher compared to patients who do not receive MV.7 Furthermore, almost 90% of episodes of nosocomial pneumonia registered in ICU occur during MV.5 The predisposing risk factors for the development of the disease are innumerable and are divided into three groups as schematized in Table 2: related with the host, the hospitalization process and with drug therapy.
Risk factors of VAP5,65,86–89
| Risk factors of VAP related with the host | Risk factors of VAP related with hospitalization process | Risk factors of VAP related with drug therapy |
|---|---|---|
| -Advanced age -Burns -Chronic or preexisting pulmonary disease (tuberculosis, chronic obstructive pulmonary disease,b bronchiolitis) -Cigarette smoking -Coma -Gastric colonization -Immunosuppressive diseasea -Impaired consciousness -Male gender -Malnutrition -Neurological/neuromuscular disease -Organ failure -Oropharynx colonization -Post-operative acute respiratory failure -Post-surgical -Post-traumatic -Septicemia -Sinusitis -Traumac -Underlying disease, and its severity | -Bronchoscopy -Emergency intubationb -Endotracheal intubation -Enteral nutrition -Frequent changes of the ventilator circuit -Gastric aspirationb -High frequency of antibiotic resistance in the hospital unit where the patient is hospitalizeda -Hospitalization ≥ 5 daysa -Long-term hospital and ICU length of stay. -Long-term intubation -Mechanical ventilation -Multiple central venous lines b -Nasogastric tube -Re-intubationc -Supine body position -Thoracic surgery -Tracheostomyc -Transportation from ICU to other hospital sites | -Antacidsc -Antibiotic therapy in the previous 90 daysa -Excessive sedation -H2-receptor antagonists -Imunosupressive drugs (corticosteroids)a -Intravenous sedativesb -Neuromuscular blockers -Prior exposure to antibiotics, particularly to third-generation cephalosporins -Proton pump inhibitors -Red blood cells transfusions (immunomodulatory effects) -Stress ulcer prophylaxis |
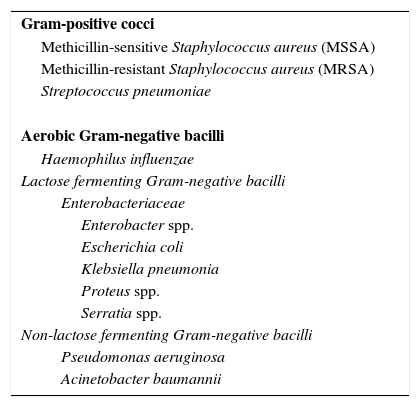
An episode of VAP may be due to a single pathogen or can have polymicrobial origin.5,8,9 The etiology of VAP is quite diverse: bacterial, fungal and viral; fungi and viruses represent a greater role when the immune system of the patients is weakened.8,10 The most common bacteria are listed in Table 3.
Predominant bacteria in ventilator-associated pneumonia.10,85,90
| Gram-positive cocci |
| Methicillin-sensitive Staphylococcus aureus (MSSA) |
| Methicillin-resistant Staphylococcus aureus (MRSA) |
| Streptococcus pneumoniae |
| Aerobic Gram-negative bacilli |
| Haemophilus influenzae |
| Lactose fermenting Gram-negative bacilli |
| Enterobacteriaceae |
| Enterobacter spp. |
| Escherichia coli |
| Klebsiella pneumonia |
| Proteus spp. |
| Serratia spp. |
| Non-lactose fermenting Gram-negative bacilli |
| Pseudomonas aeruginosa |
| Acinetobacter baumannii |
VAP is one of the major factors contributing to morbidity and mortality in the ICU.11 A meta-analysis found that the average attributable mortality to VAP is 32.5% in the ICU,12 supported by another study13 that found 33%. VAP increases the ICU and hospital length of stay, as well as the time the patient requires ventilatory support.13,14 This pathology is also responsible for more than half of the prescribed antibiotics in the ICU5 and for the increased cost of hospital internment, registering a wide range of values for the average cost attributable to the disease (between 2089.13€ and >29431.70€).14–18 Due to all this, the search for preventive measures in order to reduce these parameters, as well as to prevent the onset of the disease, has become imperative.19,20
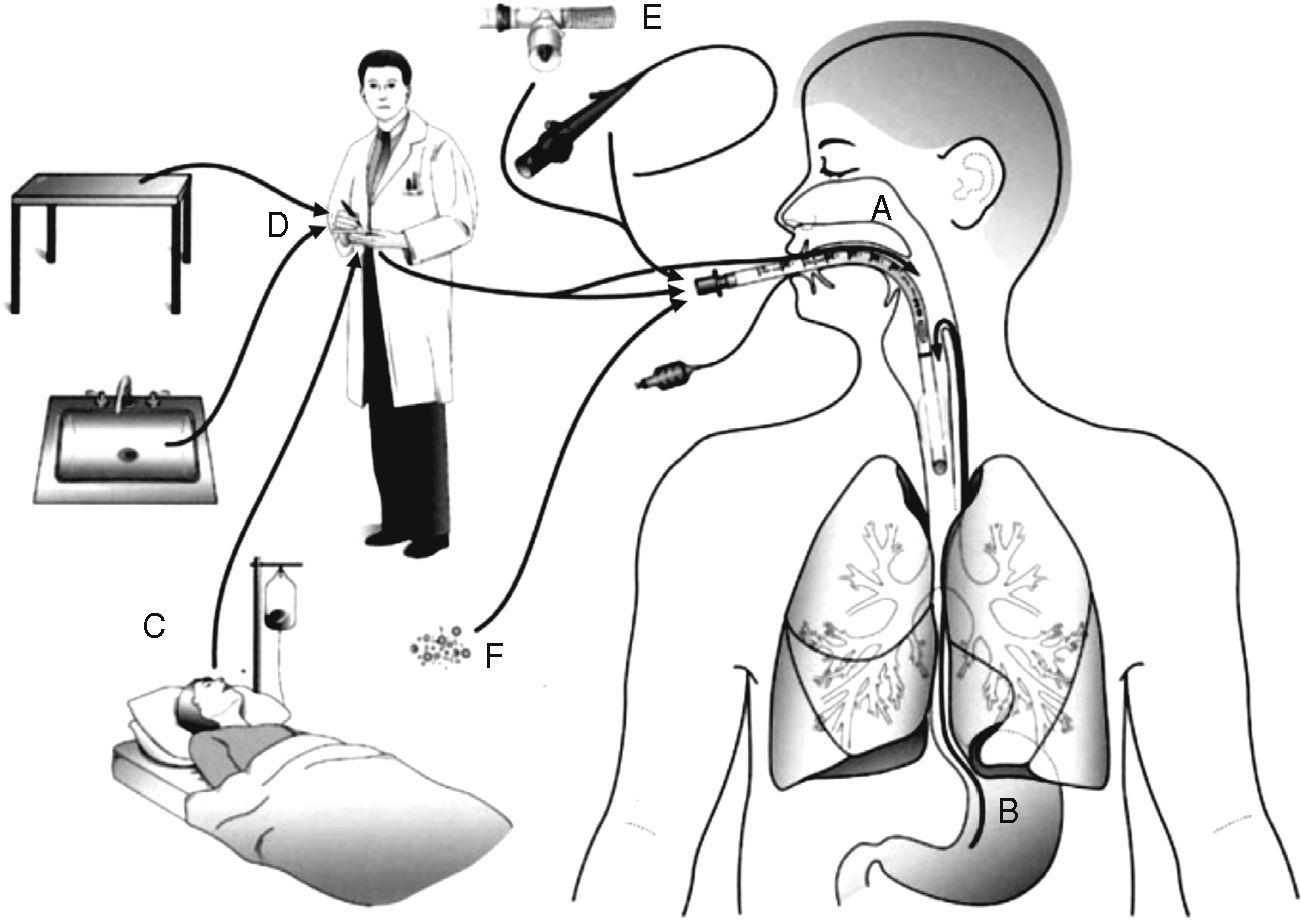
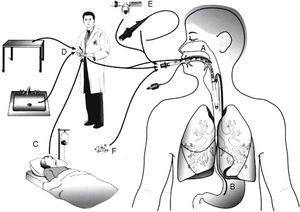
PathogenesisThere are several sources of VAP pathogens that can be classified as exogenous and endogenous in relation to the patient (Fig. 1). The exogenous sources are mostly from aerosols of the contaminated air, medical devices (humidifier, ventilatory circuit, catheter and bronchoscope), health professionals and other patients. The endogenous sources are represented by the oral, pharyngeal and gastric flora of the patient.21,22
Routes of colonization/infection in mechanically ventilated patients21 A – oral and pharyngeal colonization; B – gastric colonization; C – infected patients; D – handling of respiratory equipment; E – use of respiratory devices; and F – aerosols from contaminated air.
Microorganisms reach the lower respiratory tract mainly by microaspiration of oropharyngeal secretions or secretions that are aspirated to the oropharynx through gastric reflux; and secondarily by direct extension of a contiguous infection, inhalation of contaminated aerosols or by hematogenous spread of microorganisms from other sites of infection.21,23
The defense mechanisms against lung infection in a healthy non smoker include: the anatomy of airways, cough reflex, mucus production, mucociliary clearance, lactoferrin, basement membrane and the immune system.21,24 Not all defense mechanisms are operational in critically ill patients due to underlying diseases, sedative medication, poor nutrition and medical devices,22 such as the endotracheal tube (ETT) which is used in the MV and compromises the cough reflex and the mucociliary clearance (ETT increases mucous secretion and stagnation of secretions) and causes lesions on the surface of the tracheal epithelium.21,25 The ETT cuff prevents the aspiration of large volume secretions; however, it is not completely airtight, since there is the possibility of establishing microchannels between the tracheal mucous and the cuff when it is distended, which increases the probability of microaspiration of the accumulated secretions above the cuff (subglottic secretions) to the lower airways.25 In addition, pathogens that reach the ETT cuff are able to colonize the interior of the tube, ensuring access to the distal airways with the aid of the inspiratory flow from the MV, establishing posteriorly the lung infection.24 Previous surgeries and medication, particularly antibiotherapy, may also predispose the patient to the disease.5
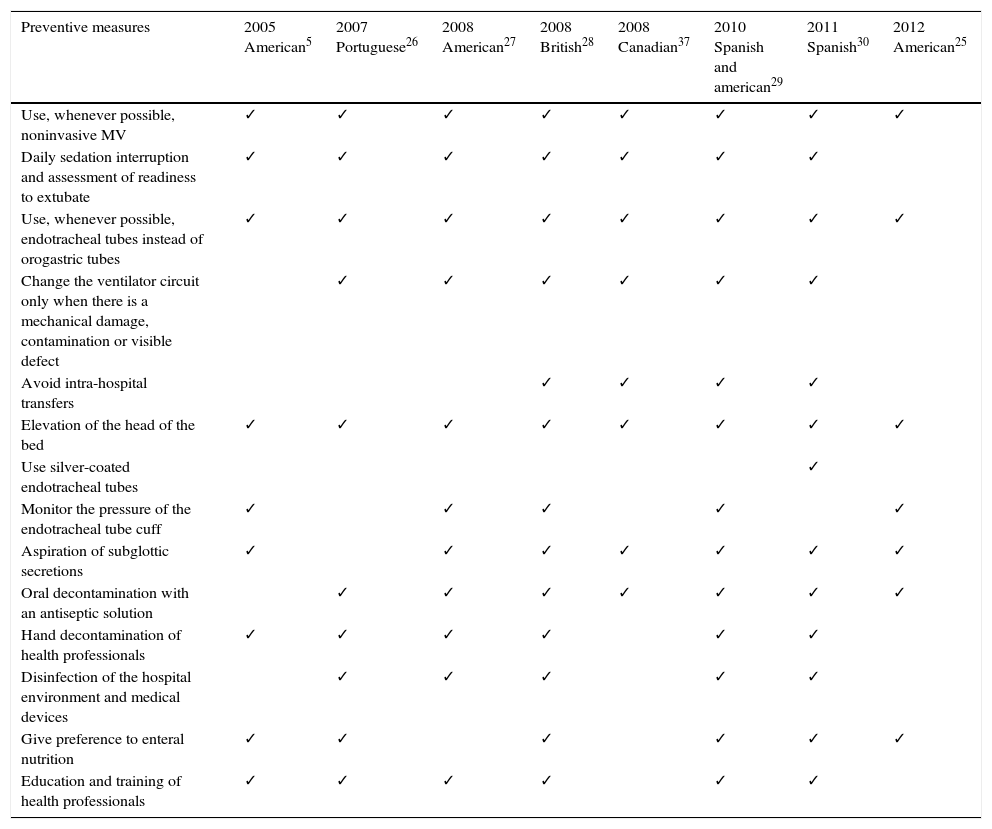
Prevention of ventilator-associated pneumoniaVAP prevention is performed through pharmacological and non-pharmacological measures that mainly focus on modifiable risk factors. With this review our intention was to approach the most consensual preventive measures that are described in guidelines drawn up by national and international scientific medical societies (Table 4 indicates the preventive measures that each guideline recommends).
Measures for prevention of VAP recommended by different guidelines.
| Preventive measures | 2005 American5 | 2007 Portuguese26 | 2008 American27 | 2008 British28 | 2008 Canadian37 | 2010 Spanish and american29 | 2011 Spanish30 | 2012 American25 |
|---|---|---|---|---|---|---|---|---|
| Use, whenever possible, noninvasive MV | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Daily sedation interruption and assessment of readiness to extubate | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Use, whenever possible, endotracheal tubes instead of orogastric tubes | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Change the ventilator circuit only when there is a mechanical damage, contamination or visible defect | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Avoid intra-hospital transfers | ✓ | ✓ | ✓ | ✓ | ||||
| Elevation of the head of the bed | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Use silver-coated endotracheal tubes | ✓ | |||||||
| Monitor the pressure of the endotracheal tube cuff | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Aspiration of subglottic secretions | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Oral decontamination with an antiseptic solution | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Hand decontamination of health professionals | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Disinfection of the hospital environment and medical devices | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Give preference to enteral nutrition | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Education and training of health professionals | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
The first step in the prevention of VAP is the patient evaluation in order to determine the need for invasive mechanical ventilation. MV should be avoided whenever possible,5,21,25–30 since, by using intubation, the risk of nosocomial pneumonia is increased 6–21 times.5
Although noninvasive MV is considered a feasible alternative to invasive MV in some clinical situations, it is important to perceive that it is not applicable in all patients, and therefore it cannot replace MV and endotracheal intubation in all situations. Noninvasive MV has been employed with favorable clinical results in cases of: exacerbation of chronic obstructive pulmonary disease (COPD), cardiogenic pulmonary edema, acute hypoxemic respiratory failure and in some immunosuppressed patients with pulmonary infiltrates and respiratory failure.5,31 A meta-analysis of 12 studies, in which the target population had COPD as the prevailing disease, concluded that noninvasive positive pressure MV, compared with invasive positive pressure MV, significantly decreased mortality and incidence of VAP, and the ICU and hospital length of stay.32
The use of noninvasive MV after extubation in order to prevent re-intubation and to reduce the time of MV (invasive and noninvasive) has been investigated and the results are now promising.33 However, more studies are needed in order to get a better understanding of its role in VAP prevention. Until now, the efficacy of this measure has only been proven in patients with COPD.31
Weaning from invasive mechanical ventilationThe weaning from invasive MV involves a close monitoring of the patient, not only because it usually occurs in an intensive care environment, but also because the monitoring of signs and symptoms is essential in identification of a possible failure of the weaning process: increased shortness of breath, tachycardia, diaphoresis, oxygen desaturation, hypertension and increased anxiety.34
Currently, the most widely used weaning method is the spontaneous breathing trial (SBT), which allows the observation for signs of respiratory failure. During SBT the patient breathes spontaneously through the ETT, which is connected to a T-piece. The T-piece can provide a source of humidified oxygen (without mechanical ventilator), reduced levels of continuous positive airway pressure and/or pressure support ventilation for short periods of time (30min–2h).34,35
Since it is difficult to predict the duration of MV, the current guidelines recommend the use of protocols for implementing the weaning process with daily assessments of the patient, in order to assess whether there are the necessary conditions to begin the weaning process.5,26–30
Ideally, protocols of invasive MV weaning include weaning from sedatives, since it was found that this procedure contributes to the reduction of the duration of MV and the ICU length of stay and, consequently, to reduce the risk of VAP.5,26–30,36 Indeed, the overuse of sedatives is not beneficial to the weaning process, as it can inhibit the patient's breathing capacity, which is indispensable to the discontinuation of MV.
Endotracheal intubationGuidelines recommend that intubation should preferably be applied with orotracheal and orogastric tubes, instead of using nasotracheal or nasogastric tubes, since oral intubation is associated with a lower incidence of VAP.5,25–29,37
Frequency of changes of the ventilator circuitOne of the risk factors related with increased hospitalization is the frequent change of the ventilator circuit. Currently it is consensual that one circuit only should be used in each patient, and that it should only be changed when there is a mechanical damage or contamination (blood, vomit, or purulent secretions).26,27,29,30,37,38 This recommendation is based on the evidence that frequent changes in the ventilator circuit do not contribute to a decreased incidence of VAP.39
Avoid unnecessary intra-hospital transfersBercault et al. found that an episode of transport from the ICU to another place in the hospital increases the risk of development of VAP.40 It is thought that the patient's positioning in supine position, and the frequent handling of the ventilator tubing during the transfers, may facilitate the aspiration of contaminated secretions.29 Nowadays, when an intra-hospital transfer is needed the patient should be in an inclined position and enteral nutrition (EN) should be suspended 4h before the transfer.28–30
Specific prevention of microaspiration- a)
Elevation of the head of the bed
Elevation of the head of the bed has the purpose of avoiding positioning the patient in supine position – a position whose leaning is 0° and is a risk factor for the development of VAP, in order to prevent gastroesophageal reflux and subsequent aspiration to the lower airways. In fact, elevation of the head of the bed is associated with a reduced risk of aspiration.27,29 A meta-analysis concluded that a head elevation of 15–30° is not sufficient to prevent the development of VAP.41 At present, elevation of the head of the bed between 30° and 45°5,25–30 is recommended, but there are already some studies and guidelines that would only consider elevation of the head of the bed at 45°.37,41 The use of the horizontal–lateral position with an elevation of 45° has shown promising results, because compared with the semi-recumbent position, it has had a lower incidence of VAP (4/10 versus 1/10).42 However, this measure is not yet included in guidelines as there are still some doubts remain to be cleared.
- b)
Modifications in endotracheal tubes
Modifications in ETT are intended to prevent mechanisms through which endotracheal intubation increases the risk of VAP: 1) aspiration of secretions into the lower airways, 2) mucosal injury and decreased mucociliary clearance of secretions, 3) microaspiration of secretions around the inflated cuff and 4) biofilm formation and bacterial colonization in the ETT lumen.43
In order to replace the traditional ETT, which have polyvinylchloride in their constitution, antibacterial-coated ETT have been developed; the most studied compound is silver, which has bactericidal activity and is able to prevent biofilm formation.44 A prospective study found that intubation with silver-coated ETT is safe, delays the ETT colonization, reduces the biofilm formation and decreases the maximal bacterial burden in tracheal secretions for 7 days.45 Another prospective study concluded that patients intubated with silver-coated ETT showed lower incidence of VAP, delay in disease onset (with higher impact in the first 10 days of intubation)46 and decreased mortality.47 Up until now, no study has reached a conclusion about the influence of this preventive measure in the duration of MV, and in ICU and hospital length of stay. Therefore, this preventive measure is still not consensual among the scientific community; so far only the Spanish guidelines recommend it.30 However, it is worth noting that a cost-effectiveness study of silver-coated ETT confirmed that, although silver-coated ETT are much more expensive (60.22€) than conventional tubes (1.47€), they reduced the hospital costs from between 7085.68€ and 12034.60€ per prevented case of VAP.48
The improvement of the ETT cuff has also been tried out, both in terms of shape as well as its inflation characteristics, in order to prevent microaspiration of subglottic secretions. The studies which analyzed ETT with polyurethane cuffs have shown good results, suggesting that they may be effective in the prevention of microaspiration of secretions to the lower airways.49–52 However, to date, none of the studies predicted the action of these tubes in the duration of MV and ICU and hospital length of stay.
Another recommendation made by some guidelines is related to the cuff pressure. It should be checked daily, at regular intervals, and maintained between 20 and 30cm H2O: below this value the risk of pneumonia increases, and above it tracheal mucosal injuries can occur.25,29 However, the British guidelines recommends a narrower range of pressure (>25 and <30cm H2O)28 and the American guidelines only recommend a minimum value of 20cm H2O.5,27
- c)
Aspiration of subglottic secretions
Currently, aspiration of subglottic secretions is recommended by almost all guidelines, since studies show a reduction in the incidence of VAP when this preventive measure is used.5,25,27–30,37 The meta-analysis of Dezfulian ital. concluded that subglottic secretion drainage reduced the incidence of VAP by almost a half, and that in patients with an estimated MV time of more than 72h this measure decreased, on average, the duration of MV by 2 days, the ICU length of stay by 3 days, and delayed the onset of pneumonia by 6.8 days.53 The recommendations concerning the use of aspiration of subglottic secretions differ between guidelines. Canadian guidelines recommend it in patients who are expected to require MV for more than 72h,37 but the Spanish have reduced the time to 48h,30 and the British recommend the implementation of this measure regardless of the expected MV duration.28 Possibly the latter approach is the most sensible, because it is very difficult to predict the duration of MV.54
There is no doubt that the use of this technique, continuously or intermittently, is effective in the prevention of VAP,55–58 but to date none of the forms of aspiration have been highlighted as the best. Currently, both are implemented in order to prevent and reduce microaspiration and, for this purpose, specific ETT have been developed. These ETT have a second lumen (aspiration lumen), which has an evacuation orifice in the edge of the cuff connected to a suction system, allowing continuous or intermittent aspiration of the subglottic secretions.25,30,59
A major aim of VAP prevention consists in reducing the colonization of the oropharynx and the digestive tract, since they represent an increased risk of microaspiration of pathogens to the lower airways.21
- a)
Oral antiseptics
Patients who are intubated with ETT cannot perform their daily oral hygiene, which carries a risk of biofilm colonization by pathogenic microorganisms.60 Oral antiseptics have been used in order to reduce and prevent oropharyngeal colonization by bacterial pathogens, and hence this is a viable alternative since it can be implemented by health care providers.
The use of oral antiseptics significantly reduces the incidence and the relative risk (RR=0.56) of VAP, suggesting that this measure is efficient in its prevention.61 Due to its very broad spectrum of action, which covers microorganisms such as Pseudomonas aeruginosa, Acinetobacter spp., and methicillin-resistant Staphylococcus aureus,62 some of the most common pathogens implicated in VAP, chlorhexidine (CHX) is the most studied antiseptic, and its efficacy in the prevention of VAP has been proven.63,64 In fact, the most recent guidelines recommend oral decontamination with CHX as a preventive measure of VAP.25–27,30,37 However, no guidelines recommend a posology for this preventive measure. Studies used concentrations of 0.12%, 0.2% and 2% of CHX administered 2, 3 or 4 times daily.62 A more recent study suggests the use of 15ml of an oral solution of 0.12% of CHX twice a day until 24h after extubation.65
The Canadian guideline is the only one that, besides recommending the use of CHX as an oral antiseptic, also recommends that the use of iodopovidone should be considered in patients with severe head injury, based on a single study performed in this patient population.37
- b)
Antibiotic prophylaxis: Selective oropharyngeal decontamination (SOD) and selective digestive decontamination (SDD)
SOD consists of enteral administration of antimicrobial agents, while in SDD the parenteral route is also used. These decontaminations are selective because the aim is to prevent the oropharyngeal and gastric colonization by aerobic Gram-negative bacilli, S. aureus and fungi species like Candida, without affecting the commensal flora.65 Antimicrobial regimens studied do not vary very much. They consist essentially in the administration four times a day of antibiotics that act only in the gastrointestinal tract, i.e. which are not absorbed (e.g., colistin, polymyxin E, tobramycin and/or amphotericin B), and antibiotics administered intravenously (e.g. cefotaxime or ciprofloxacin) in the case of SDD.54,62
de Smet et al. have conducted the largest study concerning SOD and SDD to date, they confirmed a 2.9% decrease in the mortality rate with SOD, and a 3.5% decrease with SDD. Both decontamination procedures reduced the duration of MV, ICU and hospital length of stay, daily doses of antimicrobials and the incidence of ICU-acquired bacteremia caused by S. aureus, P. aeruginosa and Enterobacteriaceae. Moreover, isolation of Gram-negative bacteria decreased considerably in patients who were treated with SOD and SDD. Authors defend the administration of DSO because it does not include widespread systemic prophylaxis with cephalosporins, and so avoids the increase of antibiotic resistance in ICU.66 However, this study has a characteristic that has prevented the generalized recommendation of SOD and SDD: antibiotic resistance recorded in the studied ICU (Holland) is considered low (<5%) when compared to the rates of antibiotic resistance in other countries such as the USA.54,65 Studies in different locations, with different rates of antibiotic resistance, are needed in order to obtain a better and more widespread perception of the long-term effect of these measures.
A later study, carried out in the same ICU, evaluated the impact of SOD and SDD on antibiotic resistance in Gram-negative bacteria, concluding that although SOD and SDD reduced mortality, both measures were associated with a gradual increase in antibiotic resistance, primarily to ceftazidime.67 Thus, American guidelines and other more recent ones do not recommend routine use of prophylactic antibiotics and SDD, due to concerns about the rising problem of antibiotic resistance.5,30,37
- c)
Probiotics
Probiotics are viable microorganisms present in sufficient number so that they can have a beneficial effect on the health of the host, which is achieved by colonization and changes of the microflora in a compartment of the host (e.g. oral cavity, gut), where they remain temporarily, favoring the growth of bacterial species beneficial to the organism, and decreasing the presence of potential pathogens.68,69
A meta-analysis including five studies that analyzed the effect of probiotics in ventilated patients, concluded that its implementation reduced the incidence of VAP, ICU length of stay and colonization of the respiratory tract by P. aeruginosa; however, no differences were observed in mortality rates and MV duration.70 Two other studies that were not included in the mentioned analysis also proved the efficacy (reduction of VAP incidence and antibiotics use) and safety of probiotics administration in the prevention of VAP.69,71 It is a fact that probiotics administration has been shown to be promising in preventing VAP, possibly representing an alternative to antibiotic prophylaxis, due to the increasing problem of resistance. However, to date, no guidelines recommend their use. More studies are needed, with more representative patient populations, in order to extrapolate the results.
Besides all specific measures to prevent VAP, guidelines also recommend one of the most basic and common preventive measures of nosocomial infections: hygiene and disinfection of the hospital environment, healthcare professionals and medical devices.5,26–30 Regarding the latter, disinfection of the respiratory equipment, removal of the condensate with the ventilator circuit closed during the procedure, and use of sterile water when rinsing the reusable devices are recommended.
Adherence to hand hygiene programs is considered one of the most important preventive measures of healthcare-associated infections,72 and also has the advantage of being cheap. The CDC Guideline for Hand Hygiene in Health-Care Settings analyzed data from observational studies concluding that the percentage of health professionals who implement the recommended procedures for hand hygiene is highly variable, with an overall average of 40%.73 In order to reduce poor adherence, training of health professionals and assessment of their performance, distribution of information leaflets and lectures are suggested.74
Enteral nutritionEN is considered a risk factor for the development of pneumonia, because it increases the risk of aspiration of the gastric content to the lower airways.5,75 However, in ventilated patients who have a critical condition, its use is unavoidable and necessary, because it avoids the development of a catabolic state28 and decreases the incidence of infectious complications and hospitalization costs.76 Additionally, ventilation time is greater when patients are fed by parenteral route.77 A large-scale study determined the impact of early (administered within the first 48h of MV) versus late EN on ICU and hospital mortality of mechanically ventilated patients. Authors concluded that early administration of EN was associated with lower rates of ICU and hospital mortality, especially in those patients with a worse health condition; however it increased the risk of development of VAP. Since the increased risk of VAP was not associated with an increased mortality, authors recommend early administration of EN, mainly in patients who are at high risk of death.78
The UK guidelines recommend that the rate and volume of EN should be adjusted to avoid gastric distension in order to prevent aspiration of gastric content to the lower airways.28 As the semi-recumbent position (30–45°) has been associated with the prevention of aspiration of gastric content, guidelines consider it essential when the patient is fed by EN, which should be administered as soon as possible.5,25,26,29,30
Education and training for health professionalsSome guidelines emphasize the importance of VAP education and training programs for health professionals who are directly involved in providing health care to patients under MV,5,26–30 since it is associated with proven efficacy in reducing the incidence of VAP by 50%.79 A questionnaire carried out with 1200 USA nurses revealed that only 82% adhere to hand-washing practices, 77% use gloves, 52% raise the head of the bed to 30–45° during the entire day and 36% aspirate the subglotic secretions; it also revealed that nurses who used a protocol of oral hygiene were associated with a greater knowledge of the disease and a higher compliance with good service practices to reduce the risk of VAP.80 There are reasons for non-compliance with guidelines by health professionals, such as disagreement with the results of the studies, patient discomfort, fear of adverse effects, lack of resources and the associated costs with preventive measures.29
BundlesA bundle consists of a small set of preventive measures with proven efficacy, which ensures more efficient prevention of the disease, compared to the sum of their individual implementation.25,54,81
Most of the hospital teams that have evaluated preventive measures for VAP, have implemented the bundle developed by the Institute for Healthcare Improvement which is composed of: elevation of the head of the bed between 30° and 45°, daily sedation interruption and assessment of readiness to extubate, daily oral care with CHX, peptic ulcer disease prophylaxis and deep vein thrombosis prophylaxis.82
A 3-year study analyzed the impact of a slight modification to this bundle (without daily oral care with CHX), verifying the decrease of hospitalization charges and the rate of VAP.83 A literature review that includes several studies that have implemented bundles to prevent VAP concluded that the bundles decrease the incidence of VAP, duration of MV, ICU length of stay, mortality and associated costs.84
There is no complete concordance between the preventive measures included in the studied bundles and the recommendations of the most recent guidelines. In fact, deep vein thrombosis prophylaxis is not indicated in any guidelines as a preventive measure of VAP and, although peptic ulcer disease prophylaxis is present in some guidelines, it is not recommended on a routine basis.5,28 Therefore, studies are needed to assess bundles that include measures recommended by the most recent guidelines, in order to evaluate if they have a synergistic effect on patient's clinical outcomes and in the decrease of VAP incidence, when implemented together.
ConclusionOver the last few years there has been a noticeable effort to develop and improve preventive measures to reduce the incidence of VAP. It is crucial that this effort continues because, although the strategies developed in recent years are promising, VAP remains a nosocomial problem which is difficult to control, with high rates of mortality, morbidity and hospital costs.
The preventive strategy of VAP focuses on modifiable risk factors, with several pharmacological and non-pharmacological measures available, which aim to reduce the risk associated with endotracheal intubation and prevent the microaspiration of pathogens to the lower airways.
Preventive measures include avoidance of endotracheal intubation and use of noninvasive MV whenever possible, preference of orotracheal and orogastric tubes, weaning of ventilation combined with weaning of sedatives, use of a single ventilator circuit per patient, use of antibacterial-coated ETT preferably with a polyurethane cuff, aspiration of subglottic secretions, patient positioning, avoidance of unnecessary intra-hospital transfers, preference for enteral nutrition, use of oral antiseptics, good hygiene practices by health professionals, and disinfection of hospital settings and medical devices.
Administration of probiotics has shown promising results, although, to date, no guidelines recommend its use. The most promising preventive measures in the near future are the development of new ETT and bundles. However, development of a bundle that contains the current recommended preventive measures, in guidelines issued by scientific medical societies, is still lacking.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.