Cardiopulmonary exercise test (CPET), which is the gold standard for the evaluation of exercise capacity, combined with exercise Doppler echocardiography (EDE) allows to specifically explore the role of right ventricular-pulmonary circulation unit in the exercise limitation. We present here the data obtained through this technique during the follow-up evaluation of COVID-19 survivors.
We evaluated consecutive patients admitted to ASST Santi Paolo e Carlo (Milan, Italy) during the first wave of the pandemic that hit Italy in February-April 2020,1 who attended the COVID-19 respiratory follow-up clinic between May and August 2020. Given the limited availability of CPET-EDE exams, due to the need of specific resources and time to perform it, we focused on patients recovering from pneumonia. Inclusion criteria considered were: 1) age >18 years, 2) previous molecular (Reverse Transcription – Polymerase Chain Reaction) diagnosis of SARS-CoV-2 infection, 3) a radiologically confirmed diagnosis of pneumonia. Exclusion criteria were the absence of a signed informed consent, acute respiratory exacerbation in the previous 4 weeks and the presence of medical conditions contraindicating CPET. The use of these data for research purposes was approved by Milan Area 1 Ethics Committee (2020/ST/407).
All patients underwent full lung function testing and chest computed tomography (CT) evaluation, as previously described.2 Echocardiography at rest was performed according to current recommendations of the American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI).3 Exercise doppler echocardiography measurements (Epic 5; Philips, Amsterdam, The Netherlands) were continuously obtained during the incremental exercise test on a semi recumbent cycle ergometer laterally tilted by 20–30° to the left. Left ventricular (LV) outflow tract diameter at rest was obtained. Exercise measured echocardiographic doppler parameters were: tricuspid annular plane systolic excursion (TAPSE), tricuspid regurgitant velocity (TRV), early mitral peak (E) and late wave (A) flow velocities, early (e′) diastolic velocities (by tissue Doppler imaging - TDI) at the septal and lateral corner of the mitral annulus. Through these parameters we obtained an estimation of cardiac output (CO) and systolic and mean pulmonary artery pressures (PASP and mPAP).4 Mitral E velocity, corrected for relaxation estimate (E/mean e′ ratio), was used to estimate LV filling pressures. Symptom-limited, incremental (ramp protocol), exercise testing was performed using the Vmax Spectra Cardiopulmonary Exercise Testing System (SensorMedics, Yorba Linda, USA). Gas exchange variables were acquired breath-by-breath.5 An arterial blood sample was collected at the peak of the exercise.
Sixteen patients (median (interquartile ranges - IQR) age 61 (56-70) years) underwent combined CPET-EDE (12 males) at a median time of 111 days (IQR 87-143) after discharge. The unbalanced gender uniformity reflects the higher incidence of pneumonia in males seen during the first wave of pandemic in Italy.1 Four patients required orotracheal intubation and mechanical invasive ventilation, 9 continuous positive airway pressure (CPAP) or non-invasive mechanical ventilation (NIMV), 2 supplemental low-flow oxygen while 1 patient was treated at home after in-hospital monitoring. One patient had a history of well controlled asthma and 5 patients had a history of systemic hypertension.
Fifteen patients (94%) still presented some degree of parenchymal involvement at CT, with mild-to-moderate impairment of diffusing lung capacity for carbon monoxide test (DLCO) (Table 1). Median peak exercise capacity was mildly reduced, with a peak oxygen consumption (peak VO2) of 74% (IQR 71-92) of predicted. No patient had a ventilatory limitation, with the slope of the relation between ventilation and carbon dioxide output during exercise (VE/VCO2 slope) presenting median values in the limit of normal and an arterial-alveolar gradient for oxygen at the limit of normal.
Baseline, cardiopulmonary exercise test and echocardiographic data.
| Baseline characteristics | |||
|---|---|---|---|
| Male/Female n (%) | 12/4 (75/25) | FEV1 %predicted | 104 (89-118) |
| BMI kg/m2 | 27.7 (25.9-31.1) | FVC %predicted | 100 (90-115) |
| Age years | 61 (56-70) | DLCO %predicted | 65 (59-82) |
| Smoking status never/current/ex-smoker (%) | 10/0/6 (62/0/38) | KCO %predicted | 77 (66-95) |
| Pack x year | 3.8 (10.0-2.0) | Alveolar Volume % predicted | 87 (70-92) |
| Time from discharge days | 111 (87-143) | CT abnormal/total n (%) | 15/16 (94%) |
| mMRC at the time of CPET (0/1/2/3/4) | 7/7/2/0/0 | %V-RPI at CT | 25 (15-35) |
| Cardiopulmonary exercise test variables | |||
| VO2 peak %predicted | 74 (71-92) | Oxygen pulse peak %pred | 91 (87-101) |
| VO2 peak absolute, ml/min/kg | 18.9 (13.6-23.0) | Breathing reserve % | 44 (32-56) |
| Work peak %predicted | 85 (72-94) | VE/VCO2 slope L/L | 27.9 (25.9-33.0) |
| Anaerobic Threshold %VO2 max predicted | 51 (45-55) | PaO2 at peak mmHg | 86 (75-90) |
| VO2/work slope ml/min/W | 9.8 (9.3-10.7) | Alveolar-arterial gradient for O2 mmHg | 36 (30-45) |
| Respiratory Exchange Ratio at peak | 1.25 (1.18-1.36) | PaCO2 at peak mmHg | 36 (32-39) |
| Heart rate reserve % | 16 (5-21) | Lactate at peak mmol/L | 6.7 (4.0-9.2) |
| Heart rate at rest bpm | 77 (65-89) | Borg scale of dyspnea at peak | 4.0 (2.5-6.5) |
| Heart rate at peak bpm | 131 (120-148) | Borg scale of perceived exertion at peak | 5.0 (3.5-6.5) |
| Echocardiographic assessment | |||
| Rest LVEF % | 60 (58-61) | Peak PASP* mmHg | 41 (36-46) |
| Rest RV/LV diameter | 0.70 (0.64-0.81) | Rest TAPSE/PASP° mm/mmHg | 0.92 (0.79-1.16) |
| Rest RV end-diastolic volume mm | 31 (27-34) | Peak TAPSE/PASP* mm/mmHg | 0.73 (0.60-0.84) |
| Rest RV fractional area change % | 46 (40-55) | mPAP/CO slope# | 1.6 (0.7-2.3) |
| Rest S wave velocity cm/s | 13 (11-16) | Rest CO L/min | 5.6 (4.6-6.7) |
| Rest TAPSE mm | 24 (19-27) | Peak CO L/min | 12.4 (10.5-14.5) |
| Peak TAPSE mm | 31 (28-35) | Rest E/e’ ratio | 7 (6-8) |
| Rest PASP° mmHg | 26 (22-28) | Peak E/e’ ratio | 8 (6-9) |
All quantitative data median (interquartile range), qualitative data as frequencies and percentages. °available for 6 patients.
available for 7 patients; BMI: Body mass index; mMRC: modified medical research council scale for dyspnea; FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity; DLCO: Diffusing capacity of the lung for carbon monoxide; KCO: carbon monoxide transfer coefficient; CT: computed tomography; %V-RPI: visual percentage of residual parenchymal involvement at chest CT; VO2: Oxygen consumption; VCO2: Carbon dioxide output; VE: Ventilation; PaO2: partial arterial pressure for oxygen; PaCO2: partial arterial pressure for carbon dioxide; TAPSE: tricuspid annular plane systolic excursion; CO: cardiac output; PASP: pulmonary artery systolic pressure; mPAP: mean pulmonary artery pressure; E: early diastolic transmitral velocity; e’: early diastolic mitral annular tissue velocity.
Doppler echocardiography showed a normal biventricular function at rest with a preserved contractile reserve of the right ventricle through the exercise and progression of cardiac output in all patients, without signs of abnormally increased filling pressure of the LV elicited by the stress. Estimation of resting PASP was possible in 6 patients, while measurement of mPAP/CO slope in 7, which resulted normal, reflecting a proportionally adequate increase in pulmonary artery pressure to the increase in CO. TAPSE/PASP ratios suggested a preserved RV length-force relationship during exercise.
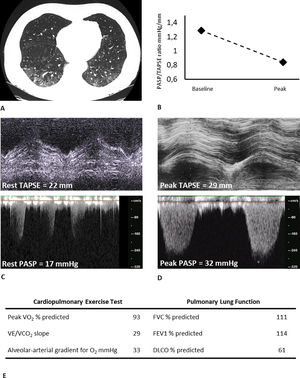
Our data add new evidence on long-term cardiopulmonary outcomes of COVID-19 survivors. Baratto et al. showed no major pathological changes in the pulmonary vascular response to exercise circulation of moderate-to-severe COVID-19 survivors at combined CPET-EDE, already at the time of hospital discharge.6 In contrast to these findings, which showed an augmented exercise hyperventilation, our data seem to confirm a recovery in time. In particular, in patients with a mild impairment in resting DLCO, an efficient vascular recruitment by cardiac output and pulmonary blood flow increase might play a prominent compensatory role7 (Fig. 1). In addition, the role of peripheral muscular function is suggested in literature to be a factor in explaining residual exercise intolerance in some patients.2,8 Systematic studies on larger samples are warranted to clarify these aspects, including stratification for severity and a specific focus on the role of the muscle.
Typical case of residual involvement at CT and DLCO, with preserved exercise capacity. A) chest CT image showing bilateral residual ground glass opacities (visual percentage of residual parenchymal involvement of 35%), B) TAPSE/PASP ratio kinetic from baseline to peak, C) Basal tricuspid annular plane systolic excursion and tricuspid regurgitant velocity, D) Peak tricuspid annular plane systolic excursion and tricuspid regurgitant velocity, E) Key parameters from CPET and PFT. FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity; DLCO: Diffusing capacity of the lung for carbon monoxide; VO2: Oxygen consumption; VCO2: Carbon dioxide output; VE: Ventilation; TAPSE: tricuspid annular plane systolic excursion; PASP: pulmonary artery systolic pressure.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
CRediT authorship contribution statementR.F. Rinaldo: Conceptualization, Data curation, Formal analysis, Project administration, Methodology, Investigation, Writing – original draft, Writing – review & editing. M. Guazzi: Conceptualization, Data curation, Methodology, Investigation, Writing – original draft, Writing – review & editing. F. Rusconi: Conceptualization, Data curation, Methodology, Investigation, Writing – original draft, Writing – review & editing. E.M. Parazzini: Conceptualization, Investigation, Writing – review & editing. F. Pitari: Data curation, Investigation, Writing – review & editing. M. Mondoni: Conceptualization, Data curation, Project administration, Investigation, Writing – review & editing. M. Balbi: Data curation, Investigation, Methodology, Writing – review & editing. F. Di Marco: Conceptualization, Methodology, Formal analysis, Supervision, Writing – original draft, Writing – review & editing. S. Centanni: Conceptualization, Methodology, Supervision, Writing – review & editing.