The baseline value of eosinophils in peripheral blood (BEC) has been associated with different degrees of severity, prognosis and response to treatment in patients with bronchiectasis. It is not known, however, if this basal value remains constant over time.
ObjectivesThe aim of this study was to assess whether the BEC remains stable in the long term in patients with bronchiectasis.
Methods and measurementsPatients from the RIBRON registry of bronchiectasis diagnosed by computed tomography with at least 2 BEC measurements one year apart were included in the study. Patients with asthma and those taking anti-eosinophilic drugs were excluded. Reliability was assessed using the intra-class correlation coefficient (ICC). A patient with a BEC of at least 300 cells/uL or less than 100 cells/uL was considered eosinophilic or eosinopenic, respectively. Group changes over time were also calculated.
Main resultsSeven hundred and thirteen patients were finally included, with a mean age of 66.5 (13.2) years (65.8 % women). A total of 2701 BEC measurements were performed, with a median number of measurements per patient of 4 (IQR: 2–5) separated by a median of 12.1 (IQR: 10.5–14.3) months between two consecutive measurements. The ICC was good (>0.75) when calculated between two consecutive measurements (approximately one year apart) but had dropped significantly by the time of the next annual measurements. Similarly, the change from an eosinophilic or eosinopenic patient to a non-eosinophilic or non-eosinopenic patient, respectively, was less than 30 % during the first year with respect to the baseline value but was close to 50 % in later measurements.
ConclusionsGiven the significant changes observed in the baseline value of the BEC over time, its monitoring is necessary in patients with bronchiectasis in order to more reliably assess its usefulness.
Bronchiectasis is a heterogeneous disease defined as an irreversible bronchial dilatation of the airways as a consequence of a vicious circle of inflammation, infection and airway remodeling. The definition of bronchiectasis should include the clinical picture characteristic of these patients. Productive cough (usually purulent) and multiple exacerbations with an infectious profile progressively cause deterioration of quality of life.1-3
Although the bronchial inflammation present in bronchiectasis is considered to have a neutrophilic profile4,5 (except in those cases associated with asthma), on many occasions other cells, particularly eosinophils and mononuclear cells, also participate in this inflammatory process.6,7
There is an important body of scientific evidence which postulates that eosinophilic inflammation is an important treatable trait in both asthma and chronic obstructive pulmonary disease (COPD),8-10 and that its presence and intensity have been related to a worse prognosis of the disease, with a greater number and severity of exacerbations and a better response to specific treatment, especially inhaled corticosteroids (ICs).11-14 However, since the assessment of bronchial inflammation involves invasive (bronchoscopic) or time-consuming (sputum cytology) tests, it has been observed that the peripheral eosinophil count (BEC) is an acceptable surrogate marker of bronchial inflammation and, above all, an acceptable measure for making therapeutic decisions.15 Some COPD guidelines consider that a BEC of at least 300 eosinophils/µL is a good cut-off point when it comes to differentiating those patients who, despite presenting a greater number of exacerbations, will respond better to ICs, while a BEC of less than 100 eosinophils/µL implies a lack of response to ICs and a greater number of adverse effects.13,14
In recent years, some studies have shown that up to 20 % of patients with bronchiectasis have a BEC of at least 300 eosinophils/µL,7,16 and that ICs could improve quality of life17 and the number and severity of exacerbations in these patients even in the absence of asthma.18 In the same way, it has been observed that both the presence of at least 300 eosinophils/µL and less than 50–100 eosinophils/µL can be associated with a greater severity of the disease and a greater number of exacerbations.7,16
One of the most important limitations when using the BEC as a surrogate marker of bronchial eosinophilic inflammation is that only its initial value is considered to analyze its association with the severity of bronchiectasis or for therapeutic decision-making.19 However, the BEC value can be affected over time by factors such as comorbidities, treatments, age, or the presence of chronic bronchial infection (CBI),20-22 which can compromise its stability over time. Disparate results have been observed in patients with COPD with respect to the repeatability of the BEC over time,22,23 but there has been only one small study of those with bronchiectasis, in 86 patients from a randomized controlled trial in which a moderate-good correlation of the BEC was observed – although this was gradually lost over time (being inferior to the initial value at 6 months) despite no effect on the BEC from the administration of ICs.24
Given the potential clinical importance of these findings for therapeutic decision-making, and the scant literature on the subject, the objective of our study was to analyze the long-term repeatability of the BEC in a large series of patients with bronchiectasis whose peripheral blood extractions were performed in the clinical stability phase.
MethodsStudy designThis was a multicentre, prospective and observational study derived from the Spanish Bronchiectasis Registry (RIBRON) involving 43 centres in Spain.25 This registry prospectively recruits general, anthropometric, radiological, etiological, microbiological, clinical, evolutive and treatment data. Patients were recruited from February 2015 to December 2019. Ethical approval was obtained from the Ethics Committee at the Hospital Josep Trueta in Girona (001–2012, Hospital Universitari Dr. Josep Trueta, Girona, Spain), in the coordinating centre and in the local participating centres. All the patients signed their informed written consent to participate in the registry.
PatientsInclusion criteria were adult patients (at least 18 years old) diagnosed with bronchiectasis by means of high-resolution computerized tomography in conditions of clinical stability (defined as at least 4 weeks free of an exacerbation period) and with at least 2 BEC measurements available, one of them being the baseline measure. Exclusion criteria included asthma, allergic bronchopulmonary aspergillosis (ABPA) and systemic corticosteroids or biological treatments for eosinophilic diseases at baseline or during follow-up. Asthma was excluded, following the recommendation of international guidelines, mainly based on lack of typical symptoms and negative complementary tests in cases of reasonable doubt (negative reversibility test, IgE levels or another complementary test to rule out ABPA).10 All blood extractions to measure BEC were performed at baseline and then annually in a stable state condition (at least four weeks without a period of exacerbation).
Statistical analysisData were tabulated using the mean (standard deviation [SD]) or median (interquartile range) for quantitative data, depending on the distribution of the variables. The normality of the distribution was analyzed using the Kolmogorov-Smirnov test. The qualitative data were tabulated according to the percentage with respect to the total value. For the comparison of independent or repeated measures, an ANOVA test with Bonferroni correction was used. In the case of qualitative variables, the chi-square test was used. The intra-class correlation coefficient (ICC) with 95 %CI was used to assess the reliability of the measures. Following Koo and Li,25 a CCI value of less than 0.50 meant poor reliability; between 0.50 and 0.75, moderate reliability, and greater than 0.75, good reliability. For terminology purposes only, in this study the terms “eosinophilic” group have been considered as those patients with a BEC of at least 300 eosinophils/µL and the “eosinopenic” group as those patients with less than 100 eosinophils/µL. The statistical packages SPSS Inc. 20 and R software were used.
ResultsOf the 2462 patients with non-cystic fibrosis bronchiectasis aged at least 18 years, 794 individuals had complete baseline data and at least two valid measurements of BEC, one of them being the baseline value. Of these, 81 patients with asthma were excluded (8 of them with ABPA, 7 taking anti-eosinophil biologic treatment and 13 taking systemic corticosteroids). Therefore, there were 713 subjects finally included in the analysis.
Baseline characteristics of the included individuals are shown in Table 1. The mean age was 66.5 (13.2) years (65.8 % women). The most frequent aetiology was post-infectious (42.7 %). The mean FEV1 % was 74.4 % (25.6) and 29.5 % presented infection by Pseudomonas aeruginosa. The mean FACED, E-FACED and BSI were 2.1 (1.6), 2.7 (2.2), and 7.2 (4.1), respectively, while 12.5 % of the patients had COPD. The mean percentage of the BEC was 3.1 % (3.12), with a mean absolute number of 202.1 (141.6) eosinophils/µL. The mean number of exacerbations during the first year after inclusion in the registry was 1.59 (1.5), and that of hospitalizations was 1.05 (1.3), with 36.7 % of the patients frequent exacerbators (at least three exacerbations during the studied year), while 62.1 % of patients used ICs (95 % from the beginning of the study and during all the follow-up).
Main baseline characteristics of the included patients, depending on the presence of peripheral eosinophilia or eosinopenia.
| Variable | All subjects(n = 713) | Baseline eosinophilia >300 eosinophils/µL(n = 124; 17.4 %) | Baseline eosinopenia <100 eosinophils/µL(n = 194; 27.2 %) |
|---|---|---|---|
| Age | 66.5 (13.2) | 66.9 (13.6) | 65.1 (11.3) |
| Gender (% females) | 65.8 % | 64.1 % | 66.2 % |
| COPD,% | 12.5 % | 12.7 % | 12.3 % |
| Smoking; pack.years | 30.3 27 | 29.8 (23.7) | 27.6 (26.3) |
| Charlson index | 1.8 (1.4) | 1.8 (1.9) | 1.8 (1.4) |
| CRP | 4.9 (11.9) | 4.2 (10.4) | 5.7 (10.4) |
| BMI, Kg/m2 | 25.7 (4.9) | 26.1 (5.1) | 25.6 (4.8) |
| FACED | 2.1 (1.6) | 1.9 (1.6) | 2.4 (1.7) |
| E-FACED | 2.7 (2.2) | 2.5 (2.3) | 3.1 (2.3) |
| BSI | 7.2 (4.1) | 6.3 (4.1) | 7.9 (4.7) |
| Previous pneumonia | 0.94 (1.7) | 0.97 (1.7) | 0.9 (1.7) |
| PA infection,% | 29.5 % | 23.2 % | 30.1 % |
| Etiology,%Post-infectiousIdiopathic | 42.7 %18.9 % | 39.4 %19.2 % | 42.3 %18.6 % |
| IgE levels, IU/mL | 61.3 (102) | 69.4 (114) | 56.3 (92) |
| FEV1,% | 74.4 (25.6) | 75.4 (25.1) | 69.4 26 |
| Exacerbationrate | 1.7 (1.6) | 1.5 (1.9) | 1.9 (1.8) |
| Hospitalization rate | 1.2 (1.6) | 1.1 (1.8) | 1.3 (1.7) |
| Exacerbator,% | 36.7 % | 33.5 % | 41.2 % |
| Eosinophils/uL | 208.5 (144) | 425.7 (123.6) | 71.6 (28.1) |
| Neutrophils,% | 58.9 % | 56.7 % | 62.5 % |
| IC treatment,% | 62.1 % | 60.3 % | 65.3 % |
| Inhaled antibiotics,% | 27 % | 24 % | 29 % |
| Macrolides,% | 26 % | 21 % | 29 % |
Data expressed as mean (standard deviation) or percentage. BSI: Bronchiectasis Severity Index; PA: Pseudomonas aeruginosa; BMI; Body Mass Index; IC: Inhaled corticosteroids; CRP: C-reactive protein.
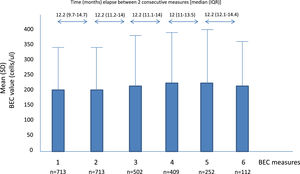
A total of 2701 BEC measurements were performed. All the patients had baseline and the second annual measurements. The median number of measurements per patients was 4 (IQR: 2–5). The median (IQR) time between two consecutive measurements was 12.1 (10.5–14.3 months) (Fig. 1). No differences were seen between the absolute number of eosinophils between measurements. However, the fourth, fifth and sixth measurements in all the cut-off points of the BEC groups analyzed changed significantly, compared with the baseline measurement. Furthermore, although there were no changes over time in the absolute number of eosinophils/µL, a significant p trend was observed towards an increase over time in the percentage of patients with a BEC >300 cells/µL or >150 cells/µL and towards a decrease in patients with BEC with less than 100 cells/µL and less than 50 cells/µL (Table 2).
Mean BEC value in absolute terms and at different cut-off points.
BEC: Blood eosinophil counts; SD: Standard deviation.
Table 3 shows the different ICCs between two consecutive BEC measurements. As can be seen, those measurements separated by no more than one year maintained a good reliability (green colour) but those separated by at least two years only had moderate reliability (yellow colour) and those separated by more than three years had poor reliability (orange colour).
Intra-class correlation coefficient between measures of blood eosinophil counts.
Green: Good reliability (>0.75); Yellow: moderate reliability (0.75–0.5) and Orange: poor reliability (<0.5).
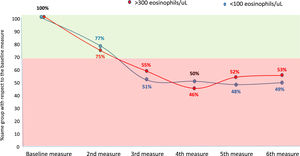
Considering the eosinophilic group as at least 300 eosinophils/µL and the eosinopenic group as less than 100 eosinophils/µL, the changes between groups were less than 30 % in the second measurement (first yearly measurement) compared with the baseline measurement. However, thereafter approximately 50 % of patients changed from an eosinophilic to a non-eosinophilic situation or from an eosinopenic to a non-eosinopenic situation respectively (Fig. 2).
DiscussionAccording to our results, although the mean absolute value of eosinophils remained constant over time in patients with bronchiectasis, the correlation between the BEC values over time, as well as the maintenance of the patient in a situation of eosinophilia (>300 eosinophils/µL) or eosinopenia (<100 eosinophils/µL), only remained constant during the first year of measurement with respect to the baseline value and decreased significantly from the second year of measurement onwards. Since BEC values could be of interest in prognostic and treatment assessment in patients with bronchiectasis, the BEC should be assessed periodically.
As occurs in patients with COPD,27-30 the relationship between the BEC values with respect to severity, prognosis or response to treatment in patients with bronchiectasis has been calculated by taking their baseline or initial value as a reference.7,16,31,32 However, it is known that BEC values can change over time due to different circumstances,21,22 so it might not be appropriate to adopt this measure without reanalyzing these values over time. It is not known, however, every time this reassessment should be done.
Nevertheless, it is remarkable that, despite the fact that the absolute values of eosinophils can fluctuate in peripheral blood for many reasons, they remained stable (on average) in our study over time in patients with bronchiectasis. This finding could have been influenced either by a statistical effect of regression to the mean by taking the measurement in a large group of patients, or by the exclusion of patients with asthma, or by the administration of systemic corticosteroid therapy or biological anti-eosinophil treatment that can lead to significant changes in the measurement of the number of peripheral eosinophils.33,34 Patients taking ICs were not excluded as these have not been shown to affect the number of peripheral eosinophils.24
However, according to our results, the correlation between the quantitative values of BEC, measured according to the ICC, is only good when the compared measurements are taken with an approximate interval of one year, while it is only moderate or poor if this interval is greater. Moreover, although the change from a situation of eosinophilia or eosinopenia to a situation of non-eosinophilia or non-eosinopenia, respectively, between the first two consecutive measurements (approximately one year apart) was less than 30 %, it rose to approximately 50 % compared to baseline beyond the first year. These results indicate that it is necessary to reassess the patient's BEC status no more than two years after the baseline measurement of peripheral eosinophils in patients with bronchiectasis.
Another interesting finding is that, despite the aforementioned stability in the overall number of eosinophils over time (with a prevalence of patients with bronchiectasis and eosinophilia and initial eosinopenia of 22 % similar to that of other series), the value of the BEC increased significantly over time, producing the opposite effect in patients with low BEC values. Thus, from the fourth BEC measurement to the sixth, the percentage of patients with eosinophilia was already significantly higher with respect to the baseline value, while that of patients with eosinopenia was significantly lower. Although the reason for this phenomenon is not clear, it could be attributed to changes in treatment intake that occur over time, although it is important to point out that all the measurements were taken during the patients' clinical stability phase.
Finally, another aspect worthy of discussion is the exclusion of patients with asthma, since it is sometimes difficult to establish the absence or presence of this disease in the presence of bronchiectasis. In our study we decided to exclude patients who presented a high probability of asthma (using the necessary complementary tests, as described in the Material and methods section) since they represented a significant source of variability and our objective was to assess whether the figures of BEC in steady-state bronchiectasis remain constant over time "per se", without being influenced by diseases or circumstances that present a tendency to suffer from peripheral eosinophilia or eosinopenia. However, the limitation of the possible inclusion of some patients who could be suffering from asthma must be recognized, although we do not believe that this circumstance would have substantially modified our conclusions.
The greatest strength of the present study is that, of all those conducted on long-term measurements of BEC in patients with bronchiectasis, it is undoubtedly the one with the largest number of patients (more than 700), the largest number of measurements (more than 2000) and the longest study period (more than 3 years). Only one other study carried out on bronchiectasis patients has observed a good correlation of the BEC, although the number of patients and the time period analyzed were low.24 We also consider that our study is representative of patients with bronchiectasis since the data have been extracted from the RIBRON registry, involving more than 40 centres throughout Spain.
Among the limitations of this study, it is important to point out that it is possible that the results cannot be extrapolated to other countries with bronchiectasis patients presenting different characteristics, since the BEC can be influenced by several intrinsic and extrinsic factors, so an external validation of the international registries would be desirable 7. Moreover, helmintic infection was not excluded. Finally, not all the patients in the RIBRON registry presented complete baseline data with repeated BEC measurements over time, especially after the 4th year of follow-up, but we believe that the high final number of measurements analyzed has allowed us to draw robust conclusions from this study.
In summary, the maintenance of patients with bronchiectasis in a situation of eosinophilia (>300 eosinophils/μL) or eosinopenia (<100 eosinophils/μL) only remained constant with respect to the baseline value during the first year of measurement and it decreased significantly thereafter. Given its clinical applicability, the BEC measurement should be assessed periodically in bronchiectasis patients. Future studies should determine the factors associated with changes in the BEC in bronchiectasis, especially those related to exacerbations,35 comorbidities,36 treatments such as ICs or antibiotics,37,38 microbiome profile39,40 and bronchial infection.41 It is also necessary to determine whether persistent peripheral eosinophilia or eosinopenia in patients with bronchiectasis are better markers of severity, prognosis or response to treatment than baseline BEC measurement alone.