Rifampicin (RFM) remains an effective treatment of pulmonary tuberculosis. Gastrointestinal adverse effects and liver toxicity are common. However, more serious reactions such as haemolytic anaemia, acute renal failure, and disseminated intravascular coagulation (DIC) have only been rarely documented. DIC secondary to RFM is a consequence of a rare immunoallergic reaction caused by the intermittent administration of RFM. It is even more uncommon in the absence of neoplastic disease, severe infection or previous exposure to RFM. Clinical features of that reaction include fever, hypotension, abdominal pain, and vomiting within hours of ingestion. Future administration of RFM is life-threatening and is contraindicated.
The aim of this report is to describe a case of RFM-induced DIC and to perform a review of the previous reports of this uncommon finding. The Pubmed® database was searched for articles in English that were published between January 1990 and January 2020. We combined search terms RFM and DIC. We also manually searched the reference lists of the eligible studies.
The authors present the case of a 68-year-old man with a history of pulmonary tuberculosis diagnosed in 2007 which was reactivated in 2018. He started the treatment with RFM, isoniazid (IZD), pyrazinamide and ethambutol. Two months later, given the clinical improvement and negative acid-fast bacilli smear, he continued the treatment with RFM and IZD. However, due to gastrointestinal symptoms (nausea and abdominal pain), the patient was following treatment intermittently (on his own initiative).
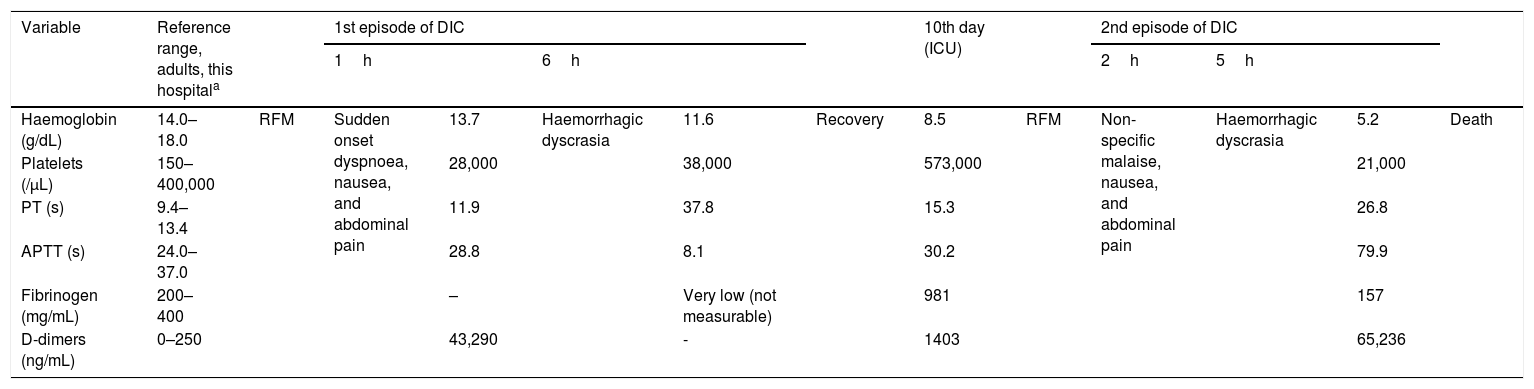
After one week of interruption, the patient returned to the treatment with RFM and IZD. One hour after, he was admitted to the Emergency Department because of a sudden onset of dyspnoea, nausea, and abdominal pain. He was in respiratory distress, febrile, hypotensive, and tachycardic. The laboratory analysis revealed low platelets (28,000/μL) and d-dimer elevation (43,290ng/ml) – Table 1.
Laboratory data. Evolution of two DIC episodes.
| Variable | Reference range, adults, this hospitala | 1st episode of DIC | 10th day (ICU) | 2nd episode of DIC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1h | 6h | 2h | 5h | ||||||||||
| Haemoglobin (g/dL) | 14.0–18.0 | RFM | Sudden onset dyspnoea, nausea, and abdominal pain | 13.7 | Haemorrhagic dyscrasia | 11.6 | Recovery | 8.5 | RFM | Non-specific malaise, nausea, and abdominal pain | Haemorrhagic dyscrasia | 5.2 | Death |
| Platelets (/μL) | 150–400,000 | 28,000 | 38,000 | 573,000 | 21,000 | ||||||||
| PT (s) | 9.4–13.4 | 11.9 | 37.8 | 15.3 | 26.8 | ||||||||
| APTT (s) | 24.0–37.0 | 28.8 | 8.1 | 30.2 | 79.9 | ||||||||
| Fibrinogen (mg/mL) | 200–400 | – | Very low (not measurable) | 981 | 157 | ||||||||
| D-dimers (ng/mL) | 0–250 | 43,290 | - | 1403 | 65,236 | ||||||||
Reference values are affected by many variables, including the patient population and the laboratory methods used. The ranges used in Hospital do Divino Espírito Santo, Ponta Delgada; are for adults who are not pregnant and do not have medical conditions that could affect the results. They may therefore not be appropriate for all patients.
Six hours after the RFM intake, the patient presented haemorrhagic dyscrasia with hemoptoic sputum, bleeding through the vascular accesses, haematuria, and haematochezia. The analytical study was compatible with DIC, showing low platelets (38,000/μL) and prolonged prothrombin time (PT) 37.8s; a prolonged activated partial thromboplastin time (APTT) 81.1s, and a very low fibrinogen level (not measurable) – Table 1. Chest angio-CT scan excluded pulmonary embolism and it revealed a ground-glass opacity infiltrate in the right lower lobe suggestive of inflammatory/infectious process.
DIC secondary to probable septic context, due to community-acquired pneumonia, was assumed. Samples were collected for cultural exams and ceftriaxone was initiated. The patient was admitted to the Intensive Care Unit (ICU).
During his stay in ICU, the patient presented good clinical and analytical recovery. On the 10th day of hospitalization, given the stability, the patient returned RFM. The analytical study (prior to RFM intake) showed no relevant changes – Table 1. Two hours after taking RFM, the patient started having nausea and abdominal pain. Five hours after RFM, a new episode of haemorrhagic dyscrasia was seen with hematemesis, bleeding through the vascular accesses and macroscopic haematuria. Hypotension refractory to fluids and vasopressor support was documented. Six hours after the RFM intake the patient presented cardio-respiratory arrest and death. Analytical study showed acute decrease in haemoglobin level (5.2g/dL) and DIC criteria with low platelets (21,000/μL), coagulopathy (PT 26.8s; APTT 79.9s; low fibrinogen 157mg/ml) and d-dimers elevation (65236ng/ml) – Table 1. Post mortem study revealed anti-RFM positive antibodies (IgM and IgG) in the patient's serum.
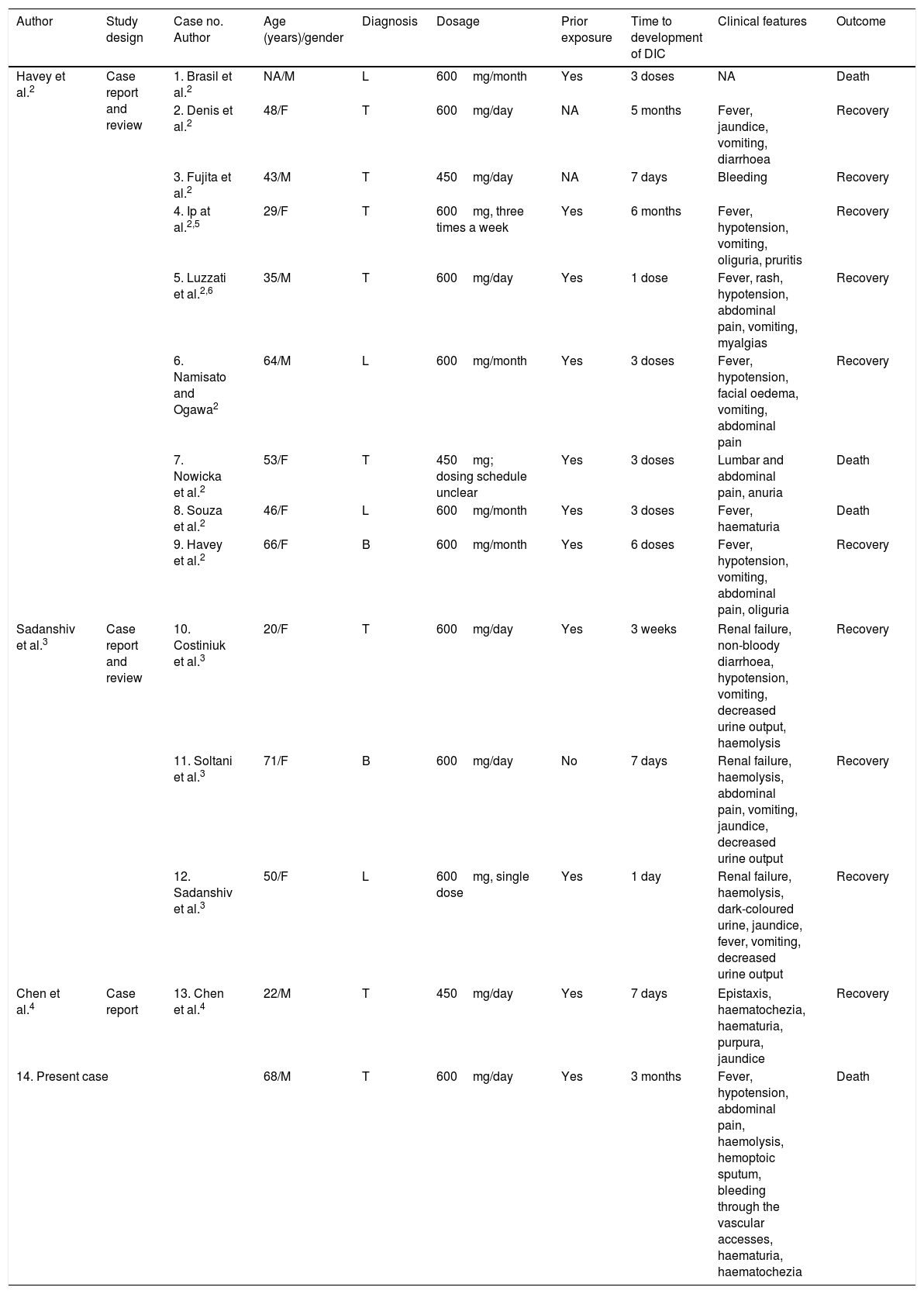
The adverse effects of RFM, include IgE-mediated allergic reactions like rashes, mild gastrointestinal disorders, hepatotoxicity, and drug interactions.1 In contrast, intermittent intake of RFM can produce more serious adverse effects induced by immunoallergic reactions mediated by IgG and IgM antibodies against erythrocytes, platelets and other target cells expressing blood antigen I, including renal tubular epithelial cells.2 Clinical manifestations of these reactions begin within hours after the ingestion of RFM and include vomiting, abdominal pain, fever, and hypotension. Diagnostic investigations generally reveal the presence of renal dysfunction, intravascular haemolysis, and DIC. The review of the existing literature identified only 13 previously reported cases of RFM-induced DIC2–4; this is the 14th case described (Table 2).
Cases of RFM-induced DIC previously described in the literature.
| Author | Study design | Case no. Author | Age (years)/gender | Diagnosis | Dosage | Prior exposure | Time to development of DIC | Clinical features | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Havey et al.2 | Case report and review | 1. Brasil et al.2 | NA/M | L | 600mg/month | Yes | 3 doses | NA | Death |
| 2. Denis et al.2 | 48/F | T | 600mg/day | NA | 5 months | Fever, jaundice, vomiting, diarrhoea | Recovery | ||
| 3. Fujita et al.2 | 43/M | T | 450mg/day | NA | 7 days | Bleeding | Recovery | ||
| 4. Ip at al.2,5 | 29/F | T | 600mg, three times a week | Yes | 6 months | Fever, hypotension, vomiting, oliguria, pruritis | Recovery | ||
| 5. Luzzati et al.2,6 | 35/M | T | 600mg/day | Yes | 1 dose | Fever, rash, hypotension, abdominal pain, vomiting, myalgias | Recovery | ||
| 6. Namisato and Ogawa2 | 64/M | L | 600mg/month | Yes | 3 doses | Fever, hypotension, facial oedema, vomiting, abdominal pain | Recovery | ||
| 7. Nowicka et al.2 | 53/F | T | 450mg; dosing schedule unclear | Yes | 3 doses | Lumbar and abdominal pain, anuria | Death | ||
| 8. Souza et al.2 | 46/F | L | 600mg/month | Yes | 3 doses | Fever, haematuria | Death | ||
| 9. Havey et al.2 | 66/F | B | 600mg/month | Yes | 6 doses | Fever, hypotension, vomiting, abdominal pain, oliguria | Recovery | ||
| Sadanshiv et al.3 | Case report and review | 10. Costiniuk et al.3 | 20/F | T | 600mg/day | Yes | 3 weeks | Renal failure, non-bloody diarrhoea, hypotension, vomiting, decreased urine output, haemolysis | Recovery |
| 11. Soltani et al.3 | 71/F | B | 600mg/day | No | 7 days | Renal failure, haemolysis, abdominal pain, vomiting, jaundice, decreased urine output | Recovery | ||
| 12. Sadanshiv et al.3 | 50/F | L | 600mg, single dose | Yes | 1 day | Renal failure, haemolysis, dark-coloured urine, jaundice, fever, vomiting, decreased urine output | Recovery | ||
| Chen et al.4 | Case report | 13. Chen et al.4 | 22/M | T | 450mg/day | Yes | 7 days | Epistaxis, haematochezia, haematuria, purpura, jaundice | Recovery |
| 14. Present case | 68/M | T | 600mg/day | Yes | 3 months | Fever, hypotension, abdominal pain, haemolysis, hemoptoic sputum, bleeding through the vascular accesses, haematuria, haematochezia | Death | ||
T – tuberculosis; B – brucellosis; L – leprosy; NA – not available.
In the case reported, as in 3 cases before, the fact that sepsis is a common source of DIC made diagnosis difficult.2 This led to the re-exposure to RFM with a new episode of DIC and a fatal outcome. 3 other fatal cases have already been documented.2 The prior treatment with RFM, the temporal association between the two episodes of DIC and the RFM intake, as well as the absence of new episodes after its interruption, pointed to the causal role of the drug in the case described. The suspicion was confirmed post mortem by the detection of anti-RFM IgG and IgM antibodies in the patient's serum. However, DIC due to RFM is a clinical diagnosis and it has been confirmed by antibodies in only two cases before.5,6 The present case is intended to draw attention to a rare side effect of a commonly used drug.
Doctor Cristina Fraga, Director of Haematology Department, Hospital do Divino Espírito Santo, Ponta Delgada, E.P.E.