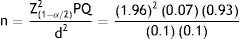Mortality of patients with pulmonary tuberculosis (TB) admitted to emergency departments is high. This study was aimed at analysing the risk factors associated with early mortality and designing a risk score based on simple parameters.
MethodsThis prospective case-control study enrolled patients admitted to the emergency department of a referral TB hospital. Clinical, radiological, biochemical and microbiological risk factors associated with death were compared among patients dying within one week from admission (cases) and those surviving (controls).
ResultsForty-nine of 250 patients (19.6%) experienced early mortality. Multiple logistic regression analysis showed that oxygen saturation (SaO2) ≤90%, severe malnutrition, tachypnoea, tachycardia, hypotension, advanced disease at chest radiography, severe anaemia, hyponatremia, hypoproteinemia and hypercapnia were independently and significantly associated with early mortality. A clinical scoring system was further designed to stratify the risk of death by selecting five simple parameters (SpO2 ≤ 90%, tachypnoea, hypotension, advanced disease at chest radiography and tachycardia). This model predicted early mortality with a positive predictive value of 94.88% and a negative predictive value of 19.90%.
ConclusionsThe scoring system based on simple parameters may help to refer severely ill patients early to a higher level to reduce mortality, improve success rates, minimise the need for pulmonary rehabilitation and prevent post-treatment sequelae.
Tuberculosis (TB) is a major public health problem worldwide. A significant number of patients die due to TB across the globe despite good control programmes in many countries including India.1 The best WHO estimate is that around 10.0 million people developed TB disease in 2018, with 1.2 million deaths from TB, including 251,000 Human Immunodeficiency Virus (HIV) positive people.1 In India the estimated number of new TB cases was 2,700,000 accounting for about a quarter of the world’s TB burden with 440,000 deaths in 2018.1–3 The India National TB Elimination Plan 2017–2025 introduced a mortality target (90% reduction by 2025, i.e. global reduction from 32 to 3 death per 100,000 population)3 to contribute to monitoring this core parameter.
The literature on factors associated with early mortality of patients with TB is scanty. The available studies are based on cohort analysis of treatment outcomes and, with a single exception,4 analysed long-term mortality with a retrospective approach.5–11 These studies identified drug-resistant TB and HIV infection as the main determinants of mortality.4–8 The risk factors for early and long-term mortality are likely to be different. Adequate identification of early mortality predictors would allow clinicians to early identify patients at risk. Moreover, as after Intensive Care Unit (ICU) admission the surviving patients often need pulmonary rehabilitation, a more precise risk stratification of these cases would be very useful, both from a clinical and public health perspective.12–16
The aims of this prospective case-control study were: 1) to identify risk factors of patients with TB associated with early mortality (within one week from admission); 2) to develop a simple risk score to be easily assessed in peripheral units to call for rapid clinical action and prevention of avoidable mortality.
MethodsThis is an international collaborative prospective case-control study consisting of two steps: 1: to identify the risk factors independently associated to early mortality; and 2: based on them, to design a simple risk score which can be applied in all health facilities to prevent early mortality.
Setting and patientsThis collaborative study was conducted between May 2017 and May 2019 at the National Institute of Tuberculosis and Respiratory Diseases, New Delhi, India (a referral, tertiary level institute for TB management) in collaboration with the Maugeri Institute, Tradate, Italy (a reference centre with interest in TB research and rehabilitation).13 In India one of four admissions to the emergency department are due to patients with diagnosed or presumptive TB. All consecutive bacteriologically confirmed (at direct sputum smear and XPERT® MTB/RIF assay) patients admitted at the emergency department with symptoms and/or signs suggestive of TB (e.g. cough, fever, or hemoptysis, for more than two weeks) were enrolled in the study upon consultation with the Maugeri Institute. The patients who died within 7 days after admission within the study period were defined as cases and those surviving were defined as controls. All causes of death were documented.
The study was approved by the ethics and research committee of the participating institutions (N° 12,007/2017 dated 07-02-2017 and 12,733/2017 dated 01-03-2017, respectively). Patients (when not possible, the relatives) signed the informed consent.
Study designAll enrolled patients underwent a comprehensive clinical examination and a detailed medical history. All the findings were entered in a clinical data-collection form, including:
- 1
Demographics and anthropometrics; socio-economic status as per Kuppuswamy scale.17; smoking history (number of bidis and cigarettes smoked per day multiplied by the year of smoking); alcohol use history according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (DSM-IV) criteria for alcohol dependence18;
- 2
Duration of signs and symptoms; history of previous anti-TB treatment; direct sputum smear for acid fast bacilli; co-morbidities (diabetes mellitus, chronic obstructive pulmonary disease, co-infection with HIV and cardiovascular diseases);
- 3
Clinical signs: arterial blood pressure, pulse rate, respiratory rate, body temperature, and presence/absence of wheezing;
- 4
Laboratory: Pulse oximetry (SpO2) and arterial blood gases; blood count, renal and liver function tests, serum electrolytes;
- 5
As per Indian guidelines all patients were tested for HIV co-infection.
- 6
All patients underwent a postero-anterior chest radiography read by two independent expert clinicians and classified according to the USA National Tuberculosis Association criteria19 as follows:
- (7)
Minimal lesions. Without cavities and of slight to moderate density. They may involve a small part of one or both lungs, but the total extent (regardless of distribution) should not exceed the volume of lung on one side that occupies the space above the second chondro-sternal junction and the spine of the forth -or body of the fifth- vertebra.
- (8)
Moderately advanced lesions. Localized in one or both lungs, cavities (if present) with a total diameter below 4 cm, with total extent not exceed the following: a. disseminated lesions of slight to moderate density throughout the total volume of one lung or the equivalent in both lungs; b. dense and confluent lesions limited in extent to one-third the volume of a single lung.
- (9)
Far advanced lesions. More extensive than the moderately advanced ones.
Other chest X-ray details (such as unilateral/ bilateral, with/without cavities) were also recorded.
Discrepancies in reading were resolved by consensus.
Statistical analysisSample size calculationPreliminary data from the emergency department (first quarter 2016) indicate that out of 175 admissions, 69 were due to bacteriologically confirmed TB patients, with high mortality within the first week following admission (5/69, 7.2%). The hypothesis guiding the study design and the sample size calculation is that a fraction of this mortality is preventable via rapid referral to higher clinical level, given adequate criteria to identify the patients are available and that a suitable cut-off to define early mortality is one week.
Therefore, considering these assumptions with 95% confidence interval and 3% absolute precision, the sample size was calculated as follows:
n = Sample size; α = 0.05; P = Percentage of patient with diagnosis of PTB; Q = (1-P); Z = 1.96 at significance level of 5%; d = Absolute precisionThe resulting sample size of 243, was rounded up to 250.
Step 1: defining risk factors for early mortalityBoth univariate and multivariate logistic regression models were used to assess the risk factors independently associated with early mortality. A p value of <0.05 was considered statistically significant.
Step 2: defining a simple risk score to predict early mortalityAmong the independent factors significantly associated to early mortality in the multiple logistic regression analysis, we identified those which could be easily evaluated in a peripheral health unit; they were used to design a clinical prediction model able to stratify the risk of death among TB patients. A specific weight was assigned to each variable based on their Odds Ratios (i.e., the risk factor with a higher odds ratio got a higher weighted score). The scores were then used to classify patients into levels of increasing risk of mortality.
The study data was analysed and the scores assigned with several simulations in such a way that no patient group with lower mortality risk would be placed at a higher level (and vice-versa). A higher risk score was therefore expected to be associated with a higher probability of early mortality.
Proportions were calculated for categorical variables and mean ± Standard Deviation (SD) for continuous variables. The data were entered in Microsoft® Excel spreadsheet and analysis was done using Statistical Package for Social Sciences (IBM, Armonk, NY, USA) version 21.0.
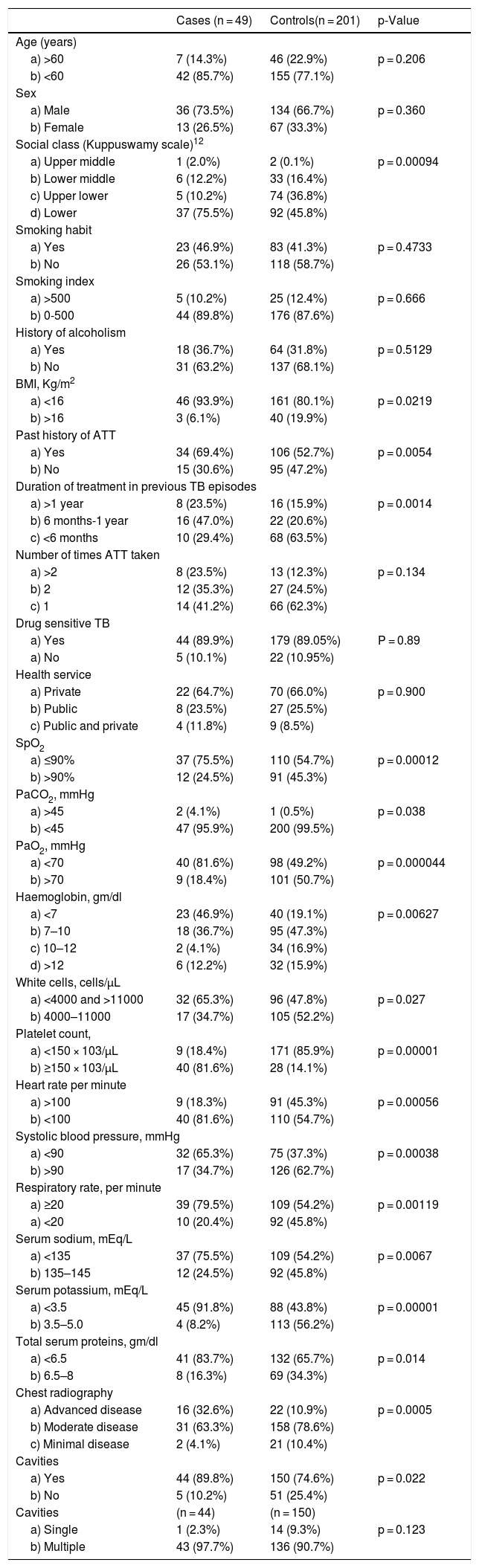
ResultsThe analysis was performed on 250 patients out of which 49 were cases (patients who died within one week from admission) and 201 controls (those who survived). Their characteristics are summarized in Table 1.
Summary of the patients’ characteristics.
| Cases (n = 49) | Controls(n = 201) | p-Value | |
|---|---|---|---|
| Age (years) | |||
| a) >60 | 7 (14.3%) | 46 (22.9%) | p = 0.206 |
| b) <60 | 42 (85.7%) | 155 (77.1%) | |
| Sex | |||
| a) Male | 36 (73.5%) | 134 (66.7%) | p = 0.360 |
| b) Female | 13 (26.5%) | 67 (33.3%) | |
| Social class (Kuppuswamy scale)12 | |||
| a) Upper middle | 1 (2.0%) | 2 (0.1%) | p = 0.00094 |
| b) Lower middle | 6 (12.2%) | 33 (16.4%) | |
| c) Upper lower | 5 (10.2%) | 74 (36.8%) | |
| d) Lower | 37 (75.5%) | 92 (45.8%) | |
| Smoking habit | |||
| a) Yes | 23 (46.9%) | 83 (41.3%) | p = 0.4733 |
| b) No | 26 (53.1%) | 118 (58.7%) | |
| Smoking index | |||
| a) >500 | 5 (10.2%) | 25 (12.4%) | p = 0.666 |
| b) 0-500 | 44 (89.8%) | 176 (87.6%) | |
| History of alcoholism | |||
| a) Yes | 18 (36.7%) | 64 (31.8%) | p = 0.5129 |
| b) No | 31 (63.2%) | 137 (68.1%) | |
| BMI, Kg/m2 | |||
| a) <16 | 46 (93.9%) | 161 (80.1%) | p = 0.0219 |
| b) >16 | 3 (6.1%) | 40 (19.9%) | |
| Past history of ATT | |||
| a) Yes | 34 (69.4%) | 106 (52.7%) | p = 0.0054 |
| b) No | 15 (30.6%) | 95 (47.2%) | |
| Duration of treatment in previous TB episodes | |||
| a) >1 year | 8 (23.5%) | 16 (15.9%) | p = 0.0014 |
| b) 6 months-1 year | 16 (47.0%) | 22 (20.6%) | |
| c) <6 months | 10 (29.4%) | 68 (63.5%) | |
| Number of times ATT taken | |||
| a) >2 | 8 (23.5%) | 13 (12.3%) | p = 0.134 |
| b) 2 | 12 (35.3%) | 27 (24.5%) | |
| c) 1 | 14 (41.2%) | 66 (62.3%) | |
| Drug sensitive TB | |||
| a) Yes | 44 (89.9%) | 179 (89.05%) | P = 0.89 |
| a) No | 5 (10.1%) | 22 (10.95%) | |
| Health service | |||
| a) Private | 22 (64.7%) | 70 (66.0%) | p = 0.900 |
| b) Public | 8 (23.5%) | 27 (25.5%) | |
| c) Public and private | 4 (11.8%) | 9 (8.5%) | |
| SpO2 | |||
| a) ≤90% | 37 (75.5%) | 110 (54.7%) | p = 0.00012 |
| b) >90% | 12 (24.5%) | 91 (45.3%) | |
| PaCO2, mmHg | |||
| a) >45 | 2 (4.1%) | 1 (0.5%) | p = 0.038 |
| b) <45 | 47 (95.9%) | 200 (99.5%) | |
| PaO2, mmHg | |||
| a) <70 | 40 (81.6%) | 98 (49.2%) | p = 0.000044 |
| b) >70 | 9 (18.4%) | 101 (50.7%) | |
| Haemoglobin, gm/dl | |||
| a) <7 | 23 (46.9%) | 40 (19.1%) | p = 0.00627 |
| b) 7–10 | 18 (36.7%) | 95 (47.3%) | |
| c) 10–12 | 2 (4.1%) | 34 (16.9%) | |
| d) >12 | 6 (12.2%) | 32 (15.9%) | |
| White cells, cells/µL | |||
| a) <4000 and >11000 | 32 (65.3%) | 96 (47.8%) | p = 0.027 |
| b) 4000–11000 | 17 (34.7%) | 105 (52.2%) | |
| Platelet count, | |||
| a) <150 × 103/µL | 9 (18.4%) | 171 (85.9%) | p = 0.00001 |
| b) ≥150 × 103/µL | 40 (81.6%) | 28 (14.1%) | |
| Heart rate per minute | |||
| a) >100 | 9 (18.3%) | 91 (45.3%) | p = 0.00056 |
| b) <100 | 40 (81.6%) | 110 (54.7%) | |
| Systolic blood pressure, mmHg | |||
| a) <90 | 32 (65.3%) | 75 (37.3%) | p = 0.00038 |
| b) >90 | 17 (34.7%) | 126 (62.7%) | |
| Respiratory rate, per minute | |||
| a) ≥20 | 39 (79.5%) | 109 (54.2%) | p = 0.00119 |
| a) <20 | 10 (20.4%) | 92 (45.8%) | |
| Serum sodium, mEq/L | |||
| a) <135 | 37 (75.5%) | 109 (54.2%) | p = 0.0067 |
| b) 135–145 | 12 (24.5%) | 92 (45.8%) | |
| Serum potassium, mEq/L | |||
| a) <3.5 | 45 (91.8%) | 88 (43.8%) | p = 0.00001 |
| b) 3.5–5.0 | 4 (8.2%) | 113 (56.2%) | |
| Total serum proteins, gm/dl | |||
| a) <6.5 | 41 (83.7%) | 132 (65.7%) | p = 0.014 |
| b) 6.5–8 | 8 (16.3%) | 69 (34.3%) | |
| Chest radiography | |||
| a) Advanced disease | 16 (32.6%) | 22 (10.9%) | p = 0.0005 |
| b) Moderate disease | 31 (63.3%) | 158 (78.6%) | |
| c) Minimal disease | 2 (4.1%) | 21 (10.4%) | |
| Cavities | |||
| a) Yes | 44 (89.8%) | 150 (74.6%) | p = 0.022 |
| b) No | 5 (10.2%) | 51 (25.4%) | |
| Cavities | (n = 44) | (n = 150) | |
| a) Single | 1 (2.3%) | 14 (9.3%) | p = 0.123 |
| b) Multiple | 43 (97.7%) | 136 (90.7%) | |
Data shown as n (%), unless otherwise stated. BMI: body mass index; ATT: anti-tuberculosis treatment; ABG: arterial blood gas; TLC: total leucocyte count; BP: blood pressure; AFB: acid fast bacilli; RBC: red blood cell; SpO2: estimate of arterial oxygen saturation PaCO2: partial pressure of oxygen in the arterial blood.
From the univariate analysis the factors significantly associated with mortality were: lower socio-economic status, longer treatment duration, severe malnutrition (assessed by Body Mass Index — BMI), previous history of anti-TB treatment, advanced disease on chest radiography, tachycardia, hypotension and tachypnoea, anaemia, sepsis, thrombocytopenia, electrolyte dis-balance including hyponatremia and hypokalemia, SpO2 ≤ 90%, hypercapnia, hypoxaemia and hypoproteinemia.
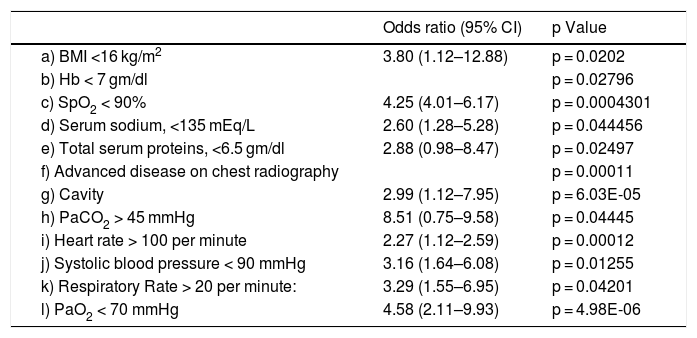
As shown in Table 2, the factors independently and significantly associated with mortality at the multiple logistic regression analysis were SpO2 ≤ 90%, severe malnutrition, tachypnea, tachycardia, hypotension, advanced disease at chest radiography, severe anaemia, hypoxaemia, hyponatremia, hypoproteinemia and hypercapnia.
Variables independently associate with early mortality at the multivariate logistic regression analysis.
| Odds ratio (95% CI) | p Value | |
|---|---|---|
| a) BMI <16 kg/m2 | 3.80 (1.12–12.88) | p = 0.0202 |
| b) Hb < 7 gm/dl | p = 0.02796 | |
| c) SpO2 < 90% | 4.25 (4.01–6.17) | p = 0.0004301 |
| d) Serum sodium, <135 mEq/L | 2.60 (1.28–5.28) | p = 0.044456 |
| e) Total serum proteins, <6.5 gm/dl | 2.88 (0.98–8.47) | p = 0.02497 |
| f) Advanced disease on chest radiography | p = 0.00011 | |
| g) Cavity | 2.99 (1.12–7.95) | p = 6.03E-05 |
| h) PaCO2 > 45 mmHg | 8.51 (0.75–9.58) | p = 0.04445 |
| i) Heart rate > 100 per minute | 2.27 (1.12–2.59) | p = 0.00012 |
| j) Systolic blood pressure < 90 mmHg | 3.16 (1.64–6.08) | p = 0.01255 |
| k) Respiratory Rate > 20 per minute: | 3.29 (1.55–6.95) | p = 0.04201 |
| l) PaO2 < 70 mmHg | 4.58 (2.11–9.93) | p = 4.98E-06 |
Abbreviations: OR: odds ratio; CI: confidence interval; BMI: Body Mass Index; SpO2: estimate of arterial oxygen saturation; PaCO2: partial pressure of oxygen in the arterial blood.
Only four patients diagnosed with TB were co-infected with HIV. Four patients were infected by rifampicin-resistant strains of Mycobacterium tuberculosis among cases and 18 among controls.
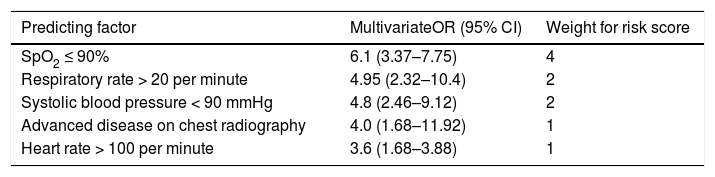
Step 2: simple risk score to predict early mortalityThe five significant risk parameters from the multivariate logistic regression analysis and easy to measure in field conditions include: SpO2 ≤90%, tachypnoea, hypotension, advanced disease at chest radiography and tachycardia (Table 3A).
Five core risk parameters composing the clinical scoring system to predict early mortality.
| Predicting factor | MultivariateOR (95% CI) | Weight for risk score |
|---|---|---|
| SpO2 ≤ 90% | 6.1 (3.37–7.75) | 4 |
| Respiratory rate > 20 per minute | 4.95 (2.32–10.4) | 2 |
| Systolic blood pressure < 90 mmHg | 4.8 (2.46–9.12) | 2 |
| Advanced disease on chest radiography | 4.0 (1.68–11.92) | 1 |
| Heart rate > 100 per minute | 3.6 (1.68–3.88) | 1 |
Abbreviations: OR: odds ratio; CI: confidence interval; SpO2: estimate of arterial oxygen saturation;
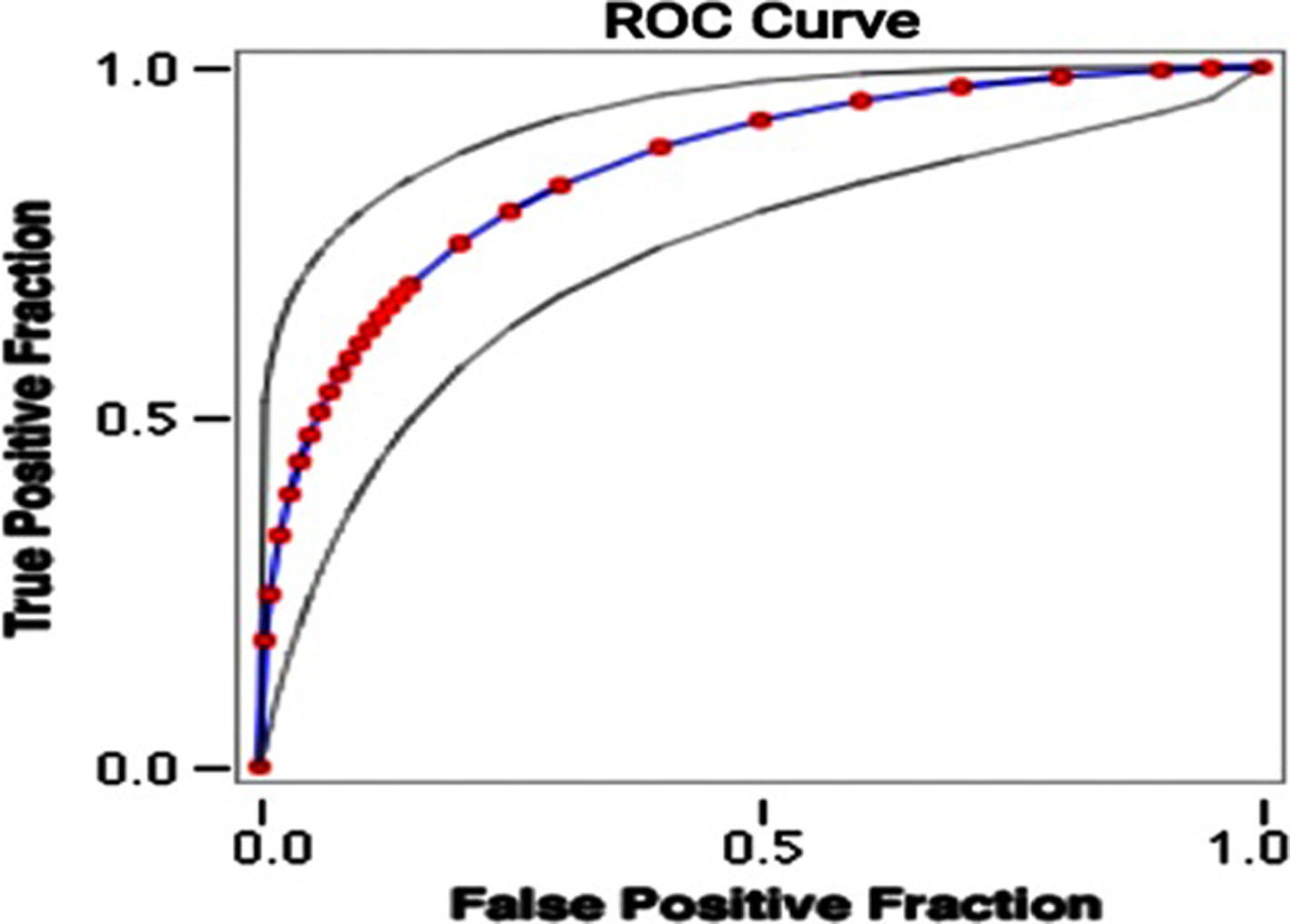
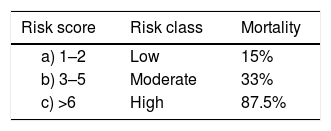
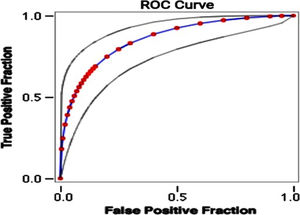
Using the weights summarised in Table 3A, the scoring system to stratify the risk of death was designed assigning a score from 0 to 6+ to each patient (Table 3B). The mortality rate associated with a risk score of 1–2, 3–5 and ≥6 was found to be 15%, 33% and 87.5%, respectively. The prediction model had area under receiver operating characteristic (ROC) curve (Fig. 1) of 0.86 indicating a very good predictability of the model. This model predicted early mortality with a positive predictive value of 94.88% and a negative predictive value of 19.90%.
This prospective study assessed the risk factors of patients with TB associated with early mortality at the emergency department level in a developing country. Furthermore, it allowed us to stratify the mortality risk by using basic and easy measurable parameters. To the best of our knowledge, our study is also the first prospective study specifically designed and powered to study early mortality of these patients admitted to the emergency department and to propose a clinical prediction model to stratify all the patients admitted to an emergency department for early mortality.9–11 Although the evidence raised is limited to India, and more evidence is needed, the model is likely to be useful in other intermediate and low-income settings, being based on simple parameters. Early referral to intensive care is likely to reduce preventable early mortality.
Our study suggests that severe malnutrition is an independent risk factor for mortality. In a study by Yung-Feng Yen et al.20 severe malnutrition was significantly associated with higher risk of all-cause mortality in these patients. The Indian Government has considered malnutrition in patients with TB as a serious concern and framed a national policy to provide financial aid to all patients by transferring cash incentives every month to their accounts till the completion of treatment as an initiative to improve their nutritional status.2 In our study severe anaemia was observed in 46.9% of cases, as compared to 19.1% of controls. Anaemia was associated with mortality in a retrospective case-control study in South Africa in which 50% of patients died.10
Interestingly enough, the factors independently and significantly associated with mortality in our study did not include history of previous treatment. As the treatment of drug-susceptible TB has not changed over the last four decades and the prevalence of drug resistance is alarming in several countries (including India), new drugs and regimens are urgently needed.21,22
The use of clinical prediction rules (CPRs) gained recent relevance in the field of pulmonary diseases. Most of the prediction rules available so far in the area of TB cover diagnosis, with only three studies providing prognosis-centred CPRs.11,23–29 Wejse et al.28 proposed the first CPR (the BandimTBscore) in a low-resource country (Guinea-Bissau), based on five symptoms and six clinical signs. However, the study included HIV-positive patients who might have been independent risk factors for mortality.24 Horita et al.30 developed a CPR to predict in-hospital TB mortality. However, exclusion of multi-drug resistant cases might have biased the conclusions as well as excluding co-morbidities (e.g. diabetes) from their prediction model.30 Bastos et al.11 conducted a retrospective cohort study in Portugal and selected five risk parameters in patients with pulmonary TB to formulate a risk assessment tool. They stratified patients into low (score 2), moderate (score 3–5) and high (score 6) mortality risk. However, six-month mortality was taken as the outcome measure.
Patients with higher scores are likely to benefit from early admission to intensive/advanced medical care units. Importantly, the prediction of mortality is independent from both the patients’ collaboration and the clinician’s subjective judgement.
Recent evidence suggests that a significant proportion of TB cases are affected by functional abnormalities (obstruction, restriction, mixed pattern) at the end of their treatment as a consequence of sequelae.12–16 Recent evidence is also available on the need for pulmonary rehabilitation following surgery for TB.31–32 A more accurate evaluation of severely ill patients with pulmonary TB with earlier access to intensive care for those for which indication exists will minimise mortality, increase the proportion of patients successfully cured and probably lower the occurrence of post-treatment sequelae. Further studies on this are needed.
Study limitationsSome demographic factors like age and sex did not match between cases and controls. Only four patients were HIV-TB co-infected, as the prevalence of HIV-TB co-infection in the Delhi population is much lower when compared to national figures.33 Therefore, the influence of this important factor on early mortality could not be properly evaluated. Furthermore, the study was conducted in a TB hospital where usually patients with advanced disease are admitted, so the mortality rates might be skewed. Furthermore, the 7 days cut-off to define early mortality (cases) is arbitrary and the proposed score needs to be tested in different settings and countries to define its generalisability.
ConclusionsThe five significant risk parameters which are independently and significantly associated with mortality in TB patients and are easy to measure in field conditions include: SpO2 ≤90%, tachypnoea, hypotension, advanced disease at chest radiography and tachycardia. We propose a scoring system to predict a mortality rate in patients with severe pulmonary TB admitted to an emergency department. The study may help clinicians at the periphery level to early identify severely ill patients using a scoring system based on simple parameters. These parameters, easily measurable in peripheral health institutions of any setting would allow early referral of such patients to specialised centres to reduce mortality, improve success rates, minimise the need for pulmonary rehabilitation and prevent post-treatment sequelae. Further studies are necessary to evaluate the workplace safety when potentially infectious TB cases are admitted, and the role of appropriate airborne infection control measures in reducing the risk of transmission to other patients, health care workers and visitors.34 Finally, although internal validation of the risk score was performed, additional studies validating the score in similar and different settings would allow us to clearly define a potentially useful prognostic score for severe TB patients.
Authors’ contributionsRS and BR conceived and drafted the manuscript with the support of GBM. All authors critically reviewed and edited the final version prior to submission.
Conflicts of interestThe authors have no conflicts of interest to declare.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
The project is part of the activities of the Global Tuberculosis Network (GTN) and of the WHO Collaborating Centre for Tuberculosis and Lung Diseases, Tradate, ITA-80, 2017-2020- GBM/RC/LDA.
The Authors wish to thank, Rosella Centis, Dina Visca (Istituti Clinici Maugeri, Tradate, Italy) and Lia D’Ambrosio (Public Health Consulting Group; Lausanne, Switzerland) for their useful comments on the manuscript.