Inhaled medication is essential for the treatment of chronic lung disease.1–3 Inhaler misuse is quite common, and it reduces medication effectiveness.4 This problem leads to poor clinical outcomes.
The aim of this study was to assess the inhalation technique of patients with asthma, chronic obstructive pulmonary disease (COPD) and asthma/COPD overlap (ACO), and to evaluate the impact of constant technique learning in every appointment.
A quasi-experimental study was conducted in the outpatient Pulmonary clinic of Faro Hospital, from September 2017 to September 2018. Patients with asthma, COPD and ACO, who were already on inhaled therapy, were included along the first six months of the study. A questionnaire was designed to evaluate patients’ demographic and clinical data, pulmonary function and inhalation technique (using pre-defined checklists). These questionnaires were filled in at three stages: at baseline, one month later and after six months. When errors were detected the correct technique was explained verbally by a physician following each assessment and a visual explanation also given.
Ninety-seven patients were included: 41.2% had asthma, 41.2% COPD and 17.5% had ACO. Table 1 describes patients’ characteristics at baseline. In total, 444 observations of inhalation technique were documented: 305 dry powder inhalers (DPI), 112 metered-dose inhalers (MDI with or without spacer) and 27 soft-mist inhalers (SMI).
Baseline sociodemographic and clinical characteristics of the participants.
| Variable | All (n=97) | Asthma (n=40) | COPD (n=40) | ACO (n=17) | p value |
|---|---|---|---|---|---|
| Age (years) | 61.2±14.3 | 53.5±16.2#1 | 69.1±8.6#1 | 60.7±10.0 | #1<0.001 |
| ≥65 years old | 44 (45.4%) | 11 (27.5%) | 27 (67.5%) | 6 (35.3%) | 0.001 |
| Male | 52 (53.6%) | 8 (20%) | 35 (87.5%) | 9 (52.9%) | 0.001 |
| Body Mass Index (kg/m2) | 28.6±6.8 | 30.9±7.8#1 | 26.6±5.4#1 | 27.9±6.3 | #10.019 |
| Level of education | |||||
| None | 4 (4.1%) | 1 (2.5%) | 2 (5%) | 1 (5.9%) | |
| Low (primary school) | 45 (46.4%) | 16 (40%) | 25 (62.5%) | 4 (23.5%) | 0.038 |
| Middle (high school) | 41(42.3%) | 18 (45%) | 11 (27.5%) | 12 (70.6%) | |
| High (bachelor or higher) | 7 (7.2%) | 5 (12.5%) | 2 (5%) | 0 (0%) | |
| Smoking status | |||||
| Non-smokers | 22 (22.7%) | 20 (50%) | 0 (0%) | 2 (11.8%) | |
| Current smokers | 36 (37.1%) | 7 (17.5%) | 18 (45%) | 11 (64.7%) | <0.001 |
| Ex-smokers | 39 (40.2%) | 13 (32.5%) | 22 (55%) | 4 (23.5%) | |
| Pack years | 38.7±25.7 | 13.4±9.1#1 #2 | 52.1±25.8#1 | 36.6±9.2 #2 | #1<0.001 |
| #20.001 | |||||
| Lung function test | |||||
| FEV1 % predicted | 69.3±25.5 | 89.5±19.4#1#2 | 54±19.7#1 | 61.7±20.0#2 | #1#2<0.001 |
| FVC % predicted | 98.5±21.4 | 108.7±20.9 | 90.1±20.2 | 96.6±16.8 | |
| FEV1/FVC ratio | 56.4±15.3 | 68.3±10.5 | 47.0±12.0 | 52.2±14.1 | <0.001 |
| mMRC | |||||
| 0 | 24 (24.7%) | 16 (40%) | 4 (10%) | 4 (23.5%) | |
| 1 | 28 (28.9%) | 14 (35%) | 8 (20%) | 6 (35.3%) | |
| 2 | 34 (35.1%) | 9 (22.5%) | 20 (50%) | 5 (29.4%) | |
| 3 | 10 (10.3%) | 1 (2.5%) | 7 (17.5%) | 2 (11.8%) | |
| 4 | 1 (1%) | 0 (0%) | 1 (2.5%) | 0 (0%) | |
| mMRC ≥2 | 45 (46.4%) | 10 (25%) | 28 (70%) | 7 (41.2%) | <0.001 |
| COPD Assessment Test | |||||
| Total score | 17.0±7.7 | ||||
| Low impact (≤10) | 8 (20%) | ||||
| Medium impact (11-20) | 18 (45%) | ||||
| High impact (21-30) | 14 (35%) | ||||
| Very high impact (31-40) | 0 (0%) | ||||
| Asthma Control Test | |||||
| Total score | 19.4±5.0 | 19.3±5.1 | |||
| Poorly controlled (5-15) | 10 (25%) | 6 (35.3%) | |||
| Not well-controlled (16-19) | 3 (7.5%) | 1 (5.9%) | |||
| Well controlled (20-25) | 27 (67.5%) | 10 (58.8%) | |||
| GOLD grade (n=59) | |||||
| 1 | 4 (10%) | 4 (23.5%) | |||
| 2 | 22 (55%) | 9 (52.9%) | |||
| 3 | 11 (27.5%) | 3 (17.6%) | |||
| 4 | 3 (7.5%) | 1 (5.9%) | |||
| GOLD group (n=59) | |||||
| A | 5 (12.5%) | 8 (47.1%) | |||
| B | 20 (50%) | 4 (23.5%) | |||
| C | 1 (2.5%) | 2 (11.8%) | |||
| D | 14 (35%) | 3 (17.6%) | |||
| GINA step of treatment (n=59) | |||||
| 1 | 1 (2.5%) | 0 (0%) | |||
| 2 | 0 (0%) | 0 (0%) | |||
| 3 | 12 (30%) | 3 (17.6%) | |||
| 4 | 21 (52.5%) | 11 (64.7%) | |||
| 5 | 6 (15%) | 3 (17.6%) | |||
| Total follow-up time (years) | 4.0±4.3 | 3.7±4.7 | 4.5±4.3 | 3.3±3.2 | ns |
| Number of inhalers | |||||
| 1 | 59 | 31 | 21 | 7 | |
| 2 | 30 | 7 | 13 | 10 | |
| 3 | 6 | 2 | 4 | 0 | |
| 4 | 2 | 0 | 2 | 0 | |
| Patients with ≥2 different devices | 38 (39.2%) | 9 (22.5%) | 19 (47.5%) | 10 (58.8%) | 0.028 |
| Type of inhaler device | |||||
| DPI | 109 | 38 | 52 | 19 | |
| MDI | 28 | 12 | 11 | 5 | |
| SMI | 8 | 1 | 4 | 3 | |
| Number of moderate to severe exacerbations in the past 12 months | 2.4±5.5 | 2.0±3.2 | 1.3±2.0 | 3.5±11.3 | ns |
| Number of exacerbations with hospitalization in the past 12 months | 0.3±0.6 | 0.2±0.4 | 0.4±0.6 | 0.3±1.0 | ns |
| Number of ER visits in the past 12 months | 1.0±4.1 | 0.6±1.0 | 0.9±1.5 | 2.4±9.4 | ns |
| Number of antibiotic treatments in the past 12 months | 0.6±1.0 | 0.6±1.1 | 0.7±0.9 | 0.3±0.7 | ns |
| Number of OCS treatments in the past 12 months | 0.6±1.0 | 0.6±1.2 | 0.5±0.8 | 0.5±1.2 | ns |
| Number of errors | 1.8±2.2 | 1.3±1.4#1 | 2.5±2.7#1 | 1.7±2.1 | #10.048 |
Data presented in number (%) and average±standard deviation. #1 Comparing asthma and COPD; #2 Comparing asthma and ACO; COPD: chronic obstructive pulmonary disease; ACO: asthma COPD overlap; ns: non-significant; FEV1: Forced Expiratory Volume in the first second; FVC: Forced Vital Capacity; mMRC: modified Medical Research Council scale; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GINA: Global Iniciative for Asthma; DPI: dry powder inhalers; MDI: metered-dose inhalers; SMI: soft-mist inhalers; ER: emergency room; OCS: oral corticosteroids.
At baseline, 69% of the patients made at least one error related to the inhaler technique. Errors were more prevalent among: ≥65 years old patients (2.5±2.8 vs 1.3±1.4 errors, p=0.021); <1 year of a total follow-up time by Pulmonology (2.9±1.6 vs 1.6±2.2 errors, p=0.002); mMRC scale ≥2 (2.5±2.6 vs 1.3±1.6 errors, p=0.002); ≥1 severe exacerbations in the previous year (2.8±2.8 vs 1.3±1.6, p=0.001); patients with low and middle education levels (53.7% and 40.3%, respectively, p=0.033) and in those using ≥1 inhaler device (84.6% had errors, p=0.007). At the outset of the study 56.7% of the patients who did not have proper inhalation techniques were more symptomatic, with a mMRC scale ≥2 (p=0.002). COPD patients had statistically more errors than the asthmatic population. Of the patients who had moderate to severe exacerbations during the year before entering the study, 90.5% misused the inhaler (p<0.001).
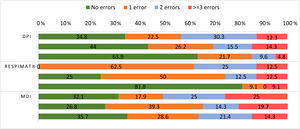
Analysing per device (Table 2), the most common errors were device-independent: not exhaling before and not breath-holding after the inhalation. The most frequent device-dependent errors were activation errors in DPI and SMI, and omitting to shake the MDI device. From the first to the last evaluation there was a significant reduction in the number of errors, especially in DPI. Fig. 1 shows the number of errors made by each patient per device type, in every evaluation. In general, there was a reduction in the number of patients who had three or more errors, and an increase in those who had no errors over the three interviews.
Description of the evolution trough the three evaluations.
| 1st evaluation (n=97) | 2nd evaluation (n=90) | 3rd evaluation (n=90) | ||||
|---|---|---|---|---|---|---|
| Difference 1st and 2nd p value | Difference 2nd and 3rd p value | Difference 1st and 3rd p value | ||||
| Number of errors | 1.9±2.3 | ns | 1.8±2.0 | 0.002 | 1.0±1.6 | <0.001 |
| Total number of devices | ||||||
| DPI | 145 | 163 | 136 | ns | ||
| - Number of devices | 109 | 99 | 97 | |||
| - Device dependent errors | 10 (0.1±0.4) | ns | 12 (0.1±0.4) | ns | 8 (0.1±0.3) | |
| - Device independent errors | 112 (1.3±1.5) | 0.023 | 77 (0.9±1.1) | <0.001 | 42 (0.5±0.8) | 0.007 |
| - Total number of errors | 122 (1.4±1.6) | 0.018 | 89 (1.1±1.3) | 0.010 | 50 (0.6±1.0) | <0.001 |
| MDI | ||||||
| - Number of devices | 28 | 56 | 28 | |||
| - Device dependent errors | 15 (0.5±0.6) | ns | 28 (0.5±0.5) | ns | 15 (0.6±0.7) | ns |
| - Device independent errors | 28 (1.0±1.1) | ns | 44 (0.8±1.0) | ns | 16 (0.6±0.9) | ns |
| - Total number of errors | 43 (1.5 ± 1.4) | ns | 72 (1.3±1.1) | ns | 31 (1.2±1.3) | ns |
| SMI | ||||||
| - Number of devices | 8 | 8 | 11 | |||
| - Device dependent errors | 2 (0.3±0.5) | -- | 0 | -- | 2 (0.3±0.7) | ns |
| - Device independent errors | 10 (1.3±0.7) | ns | 8 (1.1±1.1) | ns | 6 (0.6±1.5) | ns |
| - Total number of errors | 12 (1.5 ± 0.8) | ns | 8 (1.1±1.0) | ns | 8 (0.7 ± 2.1) | ns |
| Patients with ≥2 inhalers | 38 (39.2%) | 57 (63.3%) | 40 (44.4%) | |||
| Hospitalizations | 0.3±0.6 | <0.001 | 0.04±0.3 | ns | 0.08±0.3 | 0.001 |
| ER visits | 1.0±4.1 | <0.001 | 0.3±1.3 | 0.008 | 0.5±1.3 | ns |
| Severe exacerbations | 1.3±4.6 | <0.001 | 0.3±1.5 | 0.002 | 0.6±1.5 | 0.009 |
| Antibiotic treatments | 0.6±1.0 | <0.001 | 0.2±0.4 | 0.010 | 0.4±0.7 | ns |
| OCS treatments | 0.6±1.0 | <0.001 | 0.1±0.3 | <0.001 | 0.3±0.5 | 0.006 |
| Asthma | ||||||
| - Severe exacerbations | 0.8 ± 1.4 | 0.012 | 0.1±0.3 | ns | 0.3±0.7 | ns |
| - Hospitalizations | 0.2 ± 0.4 | 0.034 | 0±0 | ns | 0±0 | 0.020 |
| - ER visits | 0.6±1.0 | 0.018 | 0.1±0.3 | ns | 0.3±0.7 | ns |
| - Antibiotic treatment | 0.6±1.1 | 0.030 | 0.2±0.5 | ns | 0.3±0.7 | 0.050 |
| - OCS treatment | 0.6±1.2 | 0.003 | 0.1±0.2 | 0.015 | 0.2±0.4 | ns |
| - FEV1% predicted | 78.8±15.8 | -- | 81.0±21.0 | ns | ||
| - Number of errors in general | 1.3±1.4 | ns | 2.1±2.2 | 0.003 | 0.7±1.1 | 0.050 |
| - Errors in DPI | 1.0±1.0 | ns | 1.1±1.3 | 0.009 | 0.4±0.7 | 0.002 |
| - Errors in MDI | 1.1±1.1 | ns | 1.0±0.9 | ns | 1.1±1.7 | ns |
| - Errors in SMI | -- | -- | -- | |||
| COPD | ||||||
| - Severe exacerbations | 1.3±2.0 | 0.002 | 0.3±0.9 | ns | 0.6±1.2 | 0.025 |
| - Hospitalizations | 0.4±0.6 | 0.008 | 0.1±0.4 | ns | 0.1±0.4 | ns |
| - ER visits | 0.9±1.5 | 0.002 | 0.2±0.6 | 0.046 | 0.5±1.0 | ns |
| - Antibiotic treatment | 0.7±0.9 | 0.004 | 0.2±0.4 | 0.033 | 0.4±0.6 | ns |
| - OCS treatment | 0.5±0.8 | 0.001 | 0.1±0.3 | 0.005 | 0.3±0.6 | ns |
| - FEV1% predicted | 54.0±19.7 | -- | 56.5±29.6 | ns | ||
| - Number of errors in general | 2.5±2.7 | 0.011 | 1.7±1.9 | ns | 1.2±1.6 | 0.003 |
| - Errors in DPI | 1.9±2.0 | 0.001 | 1.0±1.3 | ns | 0.8±1.3 | 0.001 |
| - Errors in MDI | 1.9±1.4 | 0.016 | 1.1±0.8 | ns | 1.4±1.0 | ns |
| - Errors in SMI | 1.5±0.6 | ns | 1.3±0.5 | ns | 0.2±0.4 | ns |
| ACO | ||||||
| - Severe exacerbations | 1.3±4.5 | <0.001 | 0.3±1.5 | 0.002 | 0.6±1.5 | 0.009 |
| - Hospitalizations | 0.3±1.0 | ns | 0.1±0.2 | ns | 0.1±0.5 | ns |
| - ER visits | 2.4±9.4 | ns | 0.7±2.9 | ns | 0.9±2.5 | ns |
| - Antibiotic treatment | 0.3±0.7 | ns | 0.1±0.2 | 0.020 | 0.5±0.7 | ns |
| - OCS treatment | 0.5±1.2 | ns | 0.1±0.5 | ns | 0.3±0.6 | ns |
| - FEV1% predicted | 51.1±15.5 | -- | 53.6±16.4 | ns | ||
| - Number of errors in general | 1.6±1.9 | ns | 2.0±1.8 | ns | 1.3±2.3 | ns |
| - Errors in DPI | 0.9±1.1 | ns | 1.1±1.1 | ns | 0.6±1.1 | ns |
| - Errors in MDI | 1.8±1.8 | ns | 1.2±1.2 | ns | 1.0±1.0 | ns |
| - Errors in SMI | 1.7±1.2 | ns | 1.3±1.5 | ns | 2.3±4.0 | ns |
Data presented in number (%) and average±standard deviation. ns: non-significant; DPI: dry powder inhalers; MDI: metered-dose inhalers; SMI: soft-mist inhalers; ER: emergency room; OCS: oral corticosteroids; FEV1: Forced Expiratory Volume in the first second; COPD: chronic obstructive pulmonary disease; ACO: asthma COPD overlap.
Description of the number of errors per patient and per device type in each evaluation.
Each column represents the number (%) of errors per device type in every evaluation, from the first to the third, respectively from the top to the bottom. DPI: dry powder inhalers; MDI: metered-dose inhalers. DPI: first evaluation n=109, second evaluation n=99, third evaluation n=97; Respimat®: first evaluation n=8, second evaluation n=8, third evaluation n=11; MDI: first evaluation n=28, second evaluation n=56, third evaluation n=28.
Binary logistic regression indicates an odds ratio (OR) of 6.9 (95% CI 1.8–25.6, p=0.004) for multiple-inhalers users and an OR of 12.9 (95% CI 2.2–75.6, p=0.005) for those who make more errors. This implies a 6.9 and 12.9, respectively, increased risk of severe to moderate exacerbation in these patients, when controlling for gender, diagnosis, education level, treatment adherence, years of follow-up, symptoms and FEV1%.
This was a real-life study that demonstrated the issues related to inhaled treatment of the chronic obstructive lung diseases population: COPD patients, most of whom with a lower education status and a higher symptoms burden, showed a worse technique1; incorrect inhalation technique seems to increase with age, probably due to lung function decline and reduced hand strength and ability1,3,5; patients with at least one error had more exacerbations in the year before entering the study. These findings are consistent with studies previously published.1,2,4,6,7
Comparing the second and third evaluations, we recognize the differences between a shorter period to re-check inhalation technique (one month) against a longer one (six months). Though there was an overall improvement in both evaluations compared to the baseline, we emphasise that after a shorter period the technique and symptomatic improvement, with fewer exacerbations, was higher. These values increased slightly after five months. Therefore, this study reinforces the importance of providing regular educational training, especially within shorter periods of time, to enhance inhaler technique, ensure medication effectiveness, improve clinical management, and avoid unnecessary drug dose increments or drug modifications.1,2,4,6,7 Furthermore, this decline in exacerbations will lead to an impact in health care costs.1,5
To the best of our knowledge, there is only one Portuguese study that analysed COPD and asthma,5 which analysed a much smaller sample in two different periods. Our study included more variables and an additional evaluation, which strengthens our analysis. It shows that the positive effect of the educational intervention wanes over time stressing the need for periodic training reinforcement. Inhalation technique should, therefore, be reviewed at every appointment.
Authors contributionMarta Nobre Pereira and Vanda Areias conceived the idea and design of the study. All the authors filled in questionnaires. Marta Nobre Pereira collected the data and wrote the manuscript. Vanda Areias revised it critically for important intellectual content. All the authors read and approved the final version.
FundingThere was no funding.
Conflicts of interestThe authors declare no conflict of interest.